Thyroid Hormone Derivatives and Brain Histamine: What Should We Expect?
Annunziatina Laurino and Laura Raimondi
Annunziatina Laurino and Laura Raimondi*
Department of Neuroscience, Drug Sciences, Psychology, Child Health and Pharmacology, University of Florence, Viale Gaetano Pieraccini, Florence, Italy
- *Corresponding Author:
- Raimondi L
Department of Neuroscience
Drug Sciences, Psychology
Child Health and Pharmacology
University of Florence
Viale Gaetano Pieraccini
650139 Florence, Italy
Tel: +390552758375
E-mail: laura.raimondi@unifi.it
Received date: September 21, 2016; Accepted date: October 04, 2016; Published date: October 07, 2016
Citation: Laurino A, Raimondi L (2016) Thyroid Hormone Derivatives and Brain Histamine: What Should We Expect? J Clin Mol Endocrinol 1:26. doi: 10.21767/2572-5432.100024
Copyright: © 2016 Laurino A, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Letter to Editor
The well-recognized role of thyroid hormone (TH) is to regulate the growth, the differentiation and the metabolism of a series of body tissues including the brain by binding TH nuclear receptors. Activation of thyroid hormone receptors (α and β) initiates transcriptional activity. Several line of evidence indicate TH might also produce effects independently on activation of nuclear receptors initiating rapid “non-genomic” effects [1]. The plasma membrane integrin αvβ3 was recognized as the target responsible for such effects. Integrin binding promotes angiogenesis, tumor cell proliferation, it is involved in plasma membrane ion transporters and intracellular protein trafficking [2].
From the other side, TH metabolism could also produce compounds, at different degree of iodination, belonging to three different chemical classes including aminoacid (thyrononines), primary amines (thyronamines) and acetic or propionic acids (thyroacetic acids). Interestingly, the interconversion of compounds belonging to each of the three classes are guaranteed by the activity of enzymes including deiodinases and amine oxidases. The physiological meaning of TH metabolite tissue levels is unknown yet.
In the last years pharmacological effects of two TH metabolites, 3-iodothyronamine (T1AM) and 3-iodothyroacetic acid (TA1) have been reported in rodents. Accordingly, T1AM and TA1 emerged as potent stimulators of learning, curiosity and exploratory activity. Furthermore, T1AM and TA1 reverted druginduced amnesia and decreased pain threshold to hot stimuli [3,4].
The mechanism underlying these effects remain elusive yet. However, we reported evidence indicating that T1AM, by the production of TA1, behaves as a neuromodulator of the histaminergic system [5]. The neuromodulatory activity is a typical feature of trace amines (TA), a class of amines, as T1AM, circulating in trace in rodents and in human [6]. Collectively, pharmacological evidence reveal a link between the histaminergic system and TH metabolites. We retain such link, representing a novel finding, would merit to be further explored for its possible diagnostic but also therapeutic relevance in the context of thyroid function.
In particular, several reports indicate generalized pruritus occurring in 1–4% of patients with thyroid diseases [7] or that hypothyroid patients show a reduced pain threshold [8]. Our experimental evidence describing TA1-induced itch and thermal hyperalgesia [9] suggest that TA1 accumulation might be causally related with the onset of itch in thyroid diseases. Further, patients with thyroid dysfunctions experience sleep and mood disorders [10] and an increased incidence of seizures [11]. Consistently, histamine is among the wake-promoting neurotransmitters [12].
Accordingly, James et al. [13] reported that microinjection of T1AM in the preoptic region induced a significant reduction in non-REM sleep. Histamine is also strongly involved in mood stabilization, in the regulation of the circadian clock and of feeding behaviour. Furthermore, increasing evidence indicate a role for inflammatory mediators, including histamine and PGE2 and immune cells as principle regulators of brain sexual differentiation establishing extensive cell-to-cell communication. Brain sexual differentiation associates with different susceptibility to cellular survival following injury [14].
Pharmacological evidence suggest that drugs increasing histamine release, i.e., type 3 receptor antagonists, may have potential usefulness in ameliorating depressive status associated with sleep disorders as well as in offering neuroprotection [15]. Theoretically, a neuromodulator of the histaminergic system has the potential to reproduce pharmacological effects as those induced by an H3 receptor antagonist.
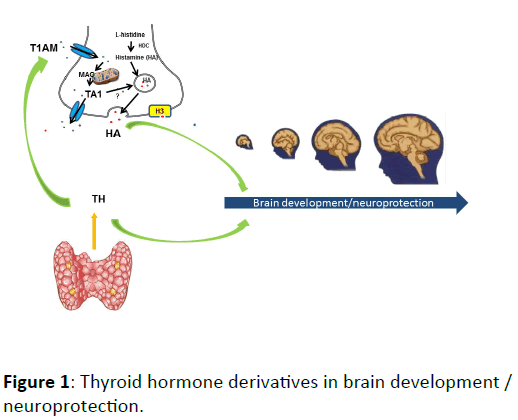
To now experimental evidence supporting this hypothesis in respect of T1AM or TA1 are lacking. However, the demonstration that T1AM or TA1 are able to reproduce central histaminergic effects, including the hormone-like effect of the amine on brain sexual differentiation, might represent a novel challenge for experimental research. In this respect, the histamine-thyroid hormone metabolites could represent the “closure of the circle” on brain differentiation initiated by TH (Figure 1).
References
- Cheng SY, Leonard JL, Davis PJ (2010) Molecular aspects of thyroid hormone actions. Endocr Rev 31: 139-170.
- Davis PJ, Davis FB, Mousa SA, Luidens MK, Lin HY (2011) Membrane receptor thyroid hormone: physiologic and pharmacologic implications. Annual review PharmacolToxicol 51:99-115.
- Manni ME, De Siena G, Saba A, Marchini M, Landucci E, et al. (2013) Pharmacological effects of 3-iodothyronamine (T1AM) in mice include facilitation of memory acquisition and retention and reduction of pain threshold. Br J Pharmacol 168: 354-362.
- Musilli C, De Siena G, Manni ME, Logli A, Landucci E, et al. (2014) Histamine mediates behavioural and metabolic effects of 3-iodothyroacetic acid, an endogenous end product of thyroid hormone metabolism. Br J Pharmacol 171: 3476-3484.
- Laurino A, De Siena G, Saba A, Chiellini G, Landucci E, et al. (2015) In the brain of mice, 3-iodothyronamine (T1AM) is converted into 3-iodothyroacetic acid (TA1) and it is included within the signaling network connecting thyroid hormone metabolites with histamine. Eur J Pharmacol 761:130-134.
- Galli E, Marchini M, Saba A, Berti S, Tonacchera M, et al. (2012) Detection of 3-iodothyronamine in human patients: a preliminary study. J ClinEndocrinolMetab 97: E69-E74.
- Ward JR, Bernhard JD (2005) Willan's itch and other causes of pruritus in the elderly. Int J Dermatol 44: 267-273.
- Guieu R, Harley JR, Blin O, Pouget J, Serratrice G (1993) Nociceptive threshold in hypothyroid patients. ActaNeurol (Napoli) 15: 183-188.
- Laurino A, De Siena G, Resta F, Masi A, Musilli C, et al. (2015) 3-iodothyroacetic acid, a metabolite of thyroid hormone, induces itch and reduces threshold to noxious and to painful heat stimuli in mice. Br J Pharmacol 172: 1859-1868.
- Smith JW, Evans AT, Costall B, Smythe JW(2002) Thyroid hormones, brain function and cognition: a brief review.NeurosciBiobehav Rev 26: 45–60.
- Song TJ, Kim SJ, Kim GS, Choi YC, Kim WJ (2010) The prevalence of thyrotoxicosis-related seizures. Thyroid 20: 955-958.
- Haas HL, Sergeeva OA, Selbach O (2008) Histamine in the nervous system. Physiol Rev 88: 1183-1241.
- James TD, Moffett SX, Scanlan TS, Martin JV (2013) Effects of acute microinjections of the thyroid hormone derivative 3-iodothyronamine to the preoptic region of adult male rats on sleep, thermoregulation and motor activity. HormBehav 64: 81–88.
- Demarest TG, McCarthy MM (2015) Sex differences in mitochondrial (dys)function: Implications for neuroprotection. J BioenergBiomembr 47: 173-188.
- Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, et al. (2015) International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacological Reviews 67: 601–655.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences