Thyroid Dysfunction Induced By Immune Check-Point Inhibitors
Ketevan Lomidze*
Department of Endocrinology, Tbilisi State Medical University, Tbilisi, Georgia
- *Corresponding Author:
- Ketevan Lomidze
Department of Endocrinology,
Tbilisi State Medical University, Tbilisi,
Georgia,
Tel: 579905003;
Email: ketilomidze86@gmail.com
Received: February 18, 2022, Manuscript No. IPJCME-22-11899; Editor assigned: February 21, 2022, PreQC No. IPJCME-22-11899 (PQ); Reviewed: March 07, 2022, QC No. IPJCME-22-11899; Revised: March 11, 2022, Manuscript No. IPJCME-22-11899 (R); Published: March 18, 2022, Invoice No. IPJCME-22-11899
Citation: Lomidze K (2022) Thyroid Dysfunction Induced By Immune Check-Point Inhibitors. J Clin Mol Endocrinol Vol:7 No:2
Abstract
The immune system is the core defense against cancer development and progression. Failure of the immune system to recognize and eliminate malignant cells plays an immense role in the pathogenesis of cancer. The paramount achievement in immunotherapy particularly Immune Checkpoint Inhibitors (ICI) over the recent decade has brought about a major paradigm shifts i n cancer treatment.
ICIs represented mainly by inhibitory monoclonal antibodies Anti-cytotoxic T-lymphocyte Antigen4 (CTLA-4) (ipilimumab), anti-programmed cell death protein 1 (PD-1- pembrolizumab/nivolumab/cemiplimab), Anti-PD-1 Ligand molecules (PD-L-1-atezolizumab/avelumab/durvalumab) reactivate the immune system against tumor c ells but can also trigger a myriad of autoimmune side effects, termed immune-related Adverse Events (irAEs). Immunotherapy related adverse events typically have a delayed onset and prolonged duration compared to adverse events from chemotherapy, and its effective management depends on early recognition and prompt intervention with immune suppression and/or immunomodulatory strategies.
The present review aims to raise awareness about the thyroid side effects of immune checkpoint inhibitors to physicians who are taking care of cancer patients and to specialists-mainly oncologists and endocrinologists who are urged to cooperate for the management of thyroid immunotoxicity.
Keywords
Immunotherapy; Antibodies; Malignant; thyroid; Prevents
Introduction
Until recently, chemotherapy, radiation, and surgery were considered the cornerstones of cancer treatment. Advances in immunotherapy have revolutionized tumor treatment. Currently, the most widely used approach is the administration of targeted Monoclonal Antibodies (mAbs) directed against T cell activation [1-5].
Under homeostatic conditions, there is a balance between pro-inflammatory and anti-inflammatory signaling maintained by immune checkpoints. These immune checkpoints are a set of inhibitory and stimulatory pathways that directly affect the function of immune cells. Malignant cells disrupt this balance by promoting an immunosuppressive state that favors immune evasion and tumor growth.
Cancer cells recruit or induce the development of regulatory T cells (Tregs), down regulate tumor antigen expression, induce T cell tolerance and/or apoptosis, and produce immune suppressive cytokines that stimulate inhibitory immune checkpoints. This leads to a unique and highly immunosuppressive Tumor Microenvironment (TME). In an attempt to overcome t hese immunosuppressive conditions, immune checkpoint inhibitors act by blocking the effects of selected inhibitory pathways. The best described immune checkpoints are CTLA-4, PD-1, and PD-L-1.
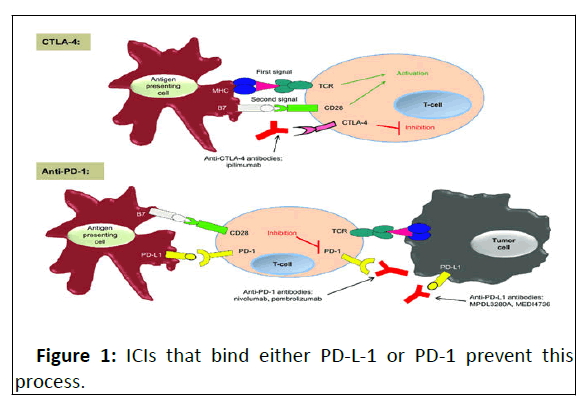
CTLA-4 is constitutively expressed by regulatory T cells and upregulated after T cell activation, acting as an “OFF” switch. CTLA-4 binds the B7 ligand on the Antigen Presenting Cells (APC). Binding CTLA-4, the immune checkpoint inhibitor prevents it from binding with B7, and allows B7 to bind with CD28, in this way inducing the immune system to attack tumor cells [6-8].
PD-1 is present on T, B, and NK cells and binds to PD-L-1, expressed by tumor cells, preventing apoptosis of the cell expressing PD-L-1 by the immune system (Figure 1).
Simplified concept of CTLA-4 and PD-1 immune checkpoints. Notes: In the priming phase, antigen-presenting cells present antigens to the T-cell. Two signals are required to initiate a T-cell response. CTLA-4 is up-regulated after T-cell activation and inhibits the T-cell response. Anti-CTLA-4 antibodies bind to CTLA-4, turning off the 'inhibitory signal', thus resulting in an enhancement of T-cell function. In the effector phase, the PD-1 inhibitory receptor is expressed by the T-cell and, when it is engaged by its ligands PD-L1 and PD-L2, it serves to inhibit the Tcell response. Anti-PD-1 antibodies bind to PD-1, turning off the 'inhibitory signal' in peripheral tissues and enhancing T-cell function. PD-1/PD-L1 interactions are complex, and this interaction is also involved in the priming phase. We have chosen to portray the main concepts for both of these immunologic checkpoints in this figure for simplicity.
Abbreviations: CD, cluster of differentiation; CTLA, cytotoxic Tlymphocyte- associated antigen; MHC, major histocompatibility complex; PD, programmed cell death; TCR, T-cell receptor.
Currently, FDA approved seven ICIs for the treatment of different solid tumors: CTLA-4 inhibitor–ipilimumab, PD-1 inhibitors–pembrolizumab, nivolumab and cemiplimab, and PDL- 1 inhibitors–atezolizumab, avelumab and durvalumab (Table 1) [9].
| TUMOR | ANTI-PD-1 | ANTI-PD-L1 | ANTI-CTLA4 | ||||
|---|---|---|---|---|---|---|---|
| PEMBROLIZUMAB | NIVOLUMAB | CEMIPLIMAB | ATEZOLIZUMAB | AVELUMAB | DURVALUMAB | IPILIMUMAB | |
| MELANOMA | V | V | V | ||||
| NSCLC | V | V | V | ||||
| HNSCC | V | V | V | V | |||
| RCC | V | V | V | V | V | ||
| UROTHELIAL | V | ||||||
| cHL | V | V | V | V | V | ||
| MSI-H | V | V | V | V | V | ||
| MCC | V | V | V | V | |||
| CSCC | V |
Table-1: summarizes the different ICIs and their main clinical indications.
NSCLC-non-small cell lung cancer, HNSCC-head and neck squamous cell carcinoma, RCC-renal cell carcinoma, cHL-classical Hodgkin’s lymphoma, MSI-H-high microsatellite instability tumor, MCC-Merkel cell carcinoma, CSCC-cutaneous squamous cell carcinoma [10].
Immune inhibitory pathways have significant roles i n the maintenance of self-tolerance, therapeutic targeting of these pathways can lead to imbalances in immunologic tolerance that manifest as immune-related Adverse Events (irAEs). A broad range of autoimmune toxicities have b een reported. Endocrine diseases are among the most commonly associated irAEs, especially immune-related thyroid dysfunctions.
The main goal of this article is to describe and analyze the incidence, pathogenesis, clinical manifestations, and guidelines on the management and screening of thyroid disorders associated with ICIs. Thus, physicians and specialists involved in treating patients can easily identify and manage immunerelated side effects [11].
Thyroid Disorders
The spectrum of thyroid disturbances under ICIs can present as thyrotoxicosis, hypothyroidism, painless thyroiditis, thyroid eye disease, and occasionally severe forms such as thyroid storm [12].
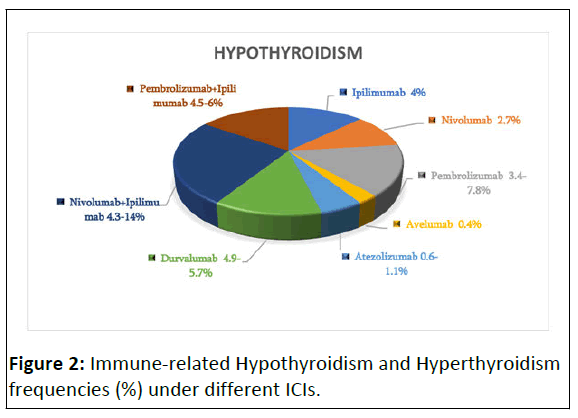
The incidence of thyroid disorders differs between different ICI classes. Chart-1 represents reported frequencies of hypothyroidism (%) and hyperthyroidism (%). Thyroid dysfunctions are mostly provoked by anti-PD-1 or anti-PD-L-1 mAbs and the incidence ranges from 4 to 19.5% (Figure 2).
The median time to onset hyperthyroidism is reported to be around 21 days in combination therapy (CTLA-4 +PD-1/PD-L-1 inhibitors) and 47 days in monotherapy with PD-1 inhibitors. For Hypothyroidism–63 days in combination therapy and 70 days in PD-1 inhibitor monotherapy, respectively. However, immunotherapy is a “living drug” and the modulation of the adaptive immune response might persist for years, resulting in immune-related thyroid dysfunction after cessation of treatment [13-15].
Although the etiology of immune-related thyroid disorders remains elusive, the knowledge that antitumor immunity and autoimmunity represent indistinguishable models of attack by T cells rationalizes the assumption that ICIs manipulating the T cells signaling toward unleashing the antitumor response, which can exacerbate autoimmunity. However, there are some data that provide support for a distinct pattern of immune-mediated thyroid destruction in autoimmune patients compared with PD-1 inhibitor (pembrolizumab) induced thyroiditis patients. In pembrolizumab-induced thyroiditis patients, there was no detectable surface expression of PD-1 on T cells. On the contrary, PD-1 expression on T cells from autoimmune patients was not different than healthy volunteer controls. As such, whereas the role of PD-1 dependent T cell activation may contribute to T cell-mediated destruction of the thyroid in pembrolizumab treated patients, the role of PD-1 in the autoimmune setting seems less likely, consistent with known antibody-mediated mechanisms for the latter [16-20].
The clinical manifestations of either hypothyroidism (bradycardia, fatigue, weight gain, constipation, dry skin, cold intolerance) or thyrotoxicosis (tachycardia, fatigue, weight loss, palpitations, new onset atrial fibrillation, diarrhea, heat intolerance, excessive diaphoresis) may be misinterpreted as symptoms of the underlying malignancy. The diagnosis of thyroid dysfunction due to ICI is based on TSH and FT4 levels to differentiate primary from central thyroid disorders. In case of thyrotoxicosis, additionally total T3 is necessary to be measured and when the etiology is unclear, thyroid scintigraphy should be performed to distinguish thyroid disruption from hyperfunction.
The handling of immune-related thyroid disorders depends on the level of TSH and the severity of symptoms. Table-2 depicts the current guidelines for the management of immune-related thyroid disorders
| irAdverse Event | ASCO Clinical Practice Guidelines | SITC Clinical Practice Guidelines | |
|---|---|---|---|
| Hypothyroidism | Grade 1: TSH, 10 mIU/L and asymptomatic | Should continue ICI with close follow-up and monitoring of TSH, FT4 | ► Thyroid function (TSH, fT4) should be tested every 4–6 weeks during ICI treatment and should continue to be tested every 6–12 months following the conclusion of ICI treatment. ► Patients with elevated TSH and normal fT4 should receive repeat TSH and fT4 testing routinely, and if this pattern persists without hypothyroidism symptoms, then levothyroxine treatment should be considered. Levothyroxine should be administered to patients with hypothyroidism at 1.5–1.6 μg/kg/day for young, healthy patients, and should be administered at 25 or 50 μg/day for patients >65 years of age or with heart disease. ► Patients with symptoms of hypothyroidism and/ or with elevated TSH and low fT4 should be tested for morning cortisol to identify possible concurrent adrenal insufficiency. ► Patients with low TSH and normal fT4 should receive repeat TSH and fT4 testing routinely, and if symptoms of hyperthyroidism or high fT4 develop patients should be treated with beta-blockers. Patients with asthma or chronic obstructive pulmonary disease should be treated with cardio selective beta-blockers such as atenolol or metoprolol. ► Patients with persistently low TSH and high fT4 should be evaluated for hyperthyroidism and Graves’ disease etiology |
| Grade 2: Moderate symptoms; able to perform ADL; TSH persistently. 10 mIU/L | May hold ICI until symptoms resolve to baseline, Consider endocrine consultation, Prescribe thyroid hormone supplementation in symptomatic patients with any degree of TSH elevation or in asymptomatic patients with TSH levels that persist. 10 mIU/L (measured 4 weeks apart) Monitor TSH every 6-8 weeks while titrating hormone replacement to normal TSH FT4 can be used in the short term (2 weeks) to ensure the adequacy of therapy in those with frank hypothyroidism where the FT4 was initially low Once adequately treated, should monitor thyroid function (at least TSH) every 6 weeks while on active ICI therapy or as needed for symptoms to ensure appropriate replacement; repeat testing annually or as indicated by symptoms once stable | ||
| Grade3-4: Severe symptoms, medically significant or life-threatening consequences, unable to perform ADL | Hold ICI until symptoms resolve to baseline with appropriate supplementation, endocrine consultation may admit for IV therapy if signs of myxedema (bradycardia, hypothermia) Thyroid supplementation and reassessment as in G2 | ||
| Additional considerations For patients without risk factors, full replacement can be estimated with an ideal body weight–based dose of approximately 1.6 mg/kg/d For elderly or fragile patients with multiple comorbidities, consider titrating up from low dose, starting at 25-50 mg Extreme elevations of TSH can be seen in the recovery phase of thyroiditis and can be watched in asymptomatic patients to determine whether there is recovery to normal within 3-4 weeks Under guidance of endocrinology, consider tapering hormone replacement and retesting in patients with a history of thyroiditis (initial thyrotoxic phase) Adrenal dysfunction, if present, must always be replaced before thyroid hormone therapy is initiated. | |||
| Hyperthyroidism | Grade1: Asymptomatic or mild symptoms | Can continue ICI with close follow-up and monitoring of TSH, FT4 every 2-3 weeks until it is clear whether there will be persistent hyperthyroidism or hypothyroidism | |
| Grade 2: Moderate symptoms, able to perform ADL | Consider holding ICI until symptoms return to baseline Consider endocrine consultation with b-Blocker (e.g., atenolol, propranolol) for symptomatic relief Hydration and supportive care Corticosteroids are not usually required to shorten duration of persistent hyperthyroidism (. 6 weeks) or clinical suspicion, work-up for Graves’ disease (TSI or TRAb) and consider thionamide (methimazole or PTU) Refer to endocrinology for Graves’ disease | ||
| Grade 3-4: Severe symptoms, medically significant or life-threatening consequences, unable to perform ADL | Hold ICI until symptoms resolve to baseline with appropriate therapy, Endocrine consultation with b-Blocker (e.g., atenolol, propranolol) for symptomatic relief for severe symptoms or concern for thyroid storm, hospitalize patient and initiate prednisone 1-2 mg/kg/d or equivalent tapered over 1-2 weeks; consider also use of SSKI or thionamide (methimazole or PTU) | ||
Table 2: Management of immunotherapy-related hyperthyroidism and hypothyroidism.
Conclusion
As check-point blockade is becoming a standard of care and several combination therapy strategies enter clinical practice, increased awareness is imperative at any time during treatment with immune checkpoint inhibitors and long after treatment cessation. Severe symptoms indicative of thyroid dysfunction require prompt intervention, while in case of nonsevere and nonspecific symptoms close monitoring is advocated. It is not well understood why some patients are more prone than others to develop endocrine side effects. Therefore, further research and investigation are needed to identify patients who are at risk for immune-related thyroid toxicity [21-24].
References
- Delivanis DA, Gustafson MP, Bornschlegl S (2017) Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights into Underlying Involved Mechanisms. J Clin Endocrinol Metab 102:2770-2780
- Marin-Acevedo JA, Kimbrough EO, Lou Y (2021) Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol 14:45
- Nakamura Y (2019) Biomarkers for Immune Checkpoint Inhibitor-Mediated Tumor Response and Adverse Events. Front Med (Lausanne) 29;6:119
- Robert C (2020) A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 11:3801
- Cukier P, Santini F C, Scaranti M, Hoff AO (2017) Endocrine side effects of cancer immunotherapy, Endocrine-Related Cancer. 24:331-347
- Puzanov I, Diab A, Abdallah K (2017) Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. j immune canc 5:95
- Maria V Deligiorgi, Mihalis I Panayiotidis & Dimitrios T Trafalis .Endocrine adverse events related with immune checkpoint inhibitors: an update for clinicians.
- El Sabbagh R, Azar NS, Eid AA, Azar ST (2020) Thyroid Dysfunctions Due to Immune Checkpoint Inhibitors: A Review. Int J Gen Med 13:1003-1009
- Hoos A (2016) Development of immuno-oncology drugs from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov 15:235–247
- Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P (2016) Harnessing the immune system to improve cancer therapy. Ann Transl Med 4:261-261
- Pardoll D (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264
- Babalola BA, Adebami GE, Akinsuyi SE (2021) Mechanistic basis for Cancer Immune Evasion and role of immune checkpoint blockades in ImmunoOncology. Glob J Cancer Ther 7:035-042
- Whiteside T (2008) The tumor microenvironment and its role in promoting tumor growth. Oncogene 27:5904–5912
- Chen L, Flies D (2013) Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13:227–242
- Joshi MN, Whitelaw BC, Palomar MTP, Wu Y (2016) Carroll PV First published: 21 March Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: clinical review Erratum in: Nat Med 23:100
- June CH, Warshauer JT, Bluestone JA (2017) Is autoimmunity the Achilles' heel of cancer immunotherapy? Nat Med 23:540-547
- Das PK, van den Wijngaard RM, Wankowicz-Kalinska A, Le Poole IC (2001) A symbiotic concept of autoimmunity and tumour immunity: lessons from vitiligo. Trends Immunol. 22:130-6
- Khan S, Gerber DE (2020) Autoimmunity, checkpoint inhibitor therapy and immune related adverse events: A review. Semin Cancer Biol 64:93-101
- Postow MA, Sidlow R, Hellmann MD (2018) Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 378:158-168
- Hargadon KM, Johnson CE, Williams CJ (2018) Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 62:29-39
- Borodic G, Hinkle DM, Cia Y (2011) Drug-induced graves’ disease from CTLA-4 receptor suppression. Ophthalmic Plast Reconstr Surg 27:87-88
- Maria Fleseriu, Ibrahim A, Hashim, Niki Karavitaki, Shlomo Melmed, (2016) Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 101:3888–3921
- Vajaitu C, Draghici CC, Solomon I (2018) The Central Role of Inflammation Associated with Checkpoint Inhibitor Treatments. J Immunol Res 4625472
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ (2018) National Comprehensive Cancer Network. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36:1714-1768
- Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC (2021) Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 9:002435
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences