Photoperiod Affects Organ Specific Glucose Metabolism in Male Siberian Hamsters (Phodopus sungorus)
Jeremy C. Borniger, Haiming Ding, Michelle Williams, Michael F. Tweedle, Michael V. Knopp, Santosh K. Maurya, Muthu Periasamy, Zachary M. Weil and Randy J. Nelson
Jeremy C. Borniger1*, Haiming Ding2, Michelle Williams2, Michael F. Tweedle2, Michael V. Knopp2, Santosh K. Maurya3, Muthu Periasamy3,4, Zachary M. Weil1 and Randy J. Nelson1
1Department of Neuroscience, Wexner Medical Center, Ohio State University
2Department of Radiology, Wexner Medical Center, Ohio State University
3Department of Physiology and Cell Biology, Davis Heart and Lung Research Institute, Columbus, OH, USA
4Davis Heart and Lung Research Institute, Columbus, OH 43210 USA
- *Corresponding Author:
- Borniger JC
Department of Neuroscience
Wexner Medical Center
Ohio State University
Columbus, OH 43210, USA
Tel: + 614-688-4674
Fax: + 614-292-3464
E-mail: Jeremy.borniger@osumc.edu
Received date: March 29, 2016; Accepted date: May 03, 2016; Published date: May 06, 2016
Citation: Borniger JC, Ding H, Williams M et al. (2016) Photoperiod Affects Organ Speci ic Glucose Metabolism in Male Siberian Hamsters (Phodopus sungorus ). J Clin Mol Endocrinol 1:7. doi: 10.21767/2572-5432.100008
Copyright: © 2016 Borniger JC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective:
Photoperiodic mammals such as Siberian hamsters (Phodopus sungorus) provide useful animal models to test metabolic plasticity. In short day (photoperiod) conditions, hamsters steadily reduce their body mass primarily through decreased white adipose tissue. Over time, hamsters reduce locomotor activity and energy expenditure in response to short days. We hypothesized that short photoperiods alter whole body and organ/tissue specific glucose metabolism. We predicted that hamsters exposed to short days would decrease whole body energy expenditure, specifically in tissues that significantly contribute to energy balance including the liver and brain.
Methods:
We collected biodistribution data after administration of the glucose analogue fluorodeoxyglucose (18F-FDG), as well as 24 h indirect calorimetry data from adult male Siberian hamsters following 10 weeks of exposure to either long (16:8) or short (8:16) photoperiods.
Results:
Short days reduced body and reproductive tissue masses, and increased splenic mass. Photoperiod influenced body mass adjusted volume of O2 consumed, heat production, and respiratory exchange ratio, with short day animals marginally reducing their energy expenditure (heat). Additionally, short-day hamsters altered the metabolic demand and efficiency of several organs, including the spleen, heart, lungs, and lower gastrointestinal system.
Conclusions:
These data suggest that short days elicit a reorganization of energy allocation from reproductive functions to somatic organ maintenance in response to short days as a winter adaptation.
Keywords
Metabolism; Photoperiod; 18F-FDG; Siberian hamsters; Indirect calorimetry
Introduction
Mechanisms have evolved that allow small mammals such as Siberian hamsters (Phodopus sungorus) to ascertain day length (photoperiod) throughout the year in order to predict seasonal changes in the environment and adjust metabolic responses accordingly [1,2]. The long days (LD) of summer and short days (SD) of winter offer different sets of ecological problems, and small mammals need to predict these challenges to optimize both reproductive fitness and survival.
Photoperiod, not variation in ambient temperature or food availability, is the primary environmental cue for time of year that promotes changes in energy requirements for thermoregulation in Siberian hamsters [3]. Individuals of this species reduce food intake, and well as body and reproductive tissue mass in preparation for the approaching winter, although photoperiod non-responsive morphs are not uncommon [4]. Decreased body mass is largely accomplished through catabolism of white adipose tissue reserves [1]. Short day exposure gradually reduces energy expenditure and elicits a loss of circadian rhythms in locomotor activity, oxygen consumption (VO2), and carbon dioxide production (VCO2) even in the absence of torpor [5]. White adipose tissue contributes significantly less to basal metabolic rate in mammals and 60% of resting energy expenditure comprises skeletal muscle, liver, and brain metabolic activity [6]. Therefore, it is likely that short days promote a reallocation of energy expenditure in these and other energetically-expensive organs.
Thus, we hypothesized that short days alter whole body energy expenditure and specific organ metabolism. We predicted that hamsters maintained in short day conditions for 10 weeks would reduce whole body energy expenditure and decrease glucose metabolism in the brain and liver compared to their long day counterparts. Reallocation of energy to specific organs in response to photoperiod may reflect important life-history trade-offs critical for survival under different day length conditions.
Methods
Animals and Photoperiodic Conditions
Three groups of adult (> 60 days old) male Siberian hamsters (Phodopus sungorus) were maintained in either short day (8:16) or long day (16:8) light-dark cycles for 10 weeks at a maintained temperature of 22 ± 2°C, which is ~4°C below the thermoneutral zone for this species [3,7]. ‘Lights-on’ was 2300 and 0700 for long and short day hamsters, respectively. ‘Lights-off’ was 1500 for hamsters in both photoperiodic conditions. Hamsters were group-housed (2-4 per cage) in polypropylene cages (32 × 18 × 14 cm), and provided with food (Harlan-Teklad no. 8640; Harlan Laboratories, Indianapolis, IN, USA) and filtered tap water ad libitum. Although group housing influences body mass and torpor dynamics in Siberian hamsters, it does not influence body-mass adjusted rate of oxygen consumption [8]. Cages were changed and body mass measures were obtained once per week between 1000 and 1200. After 10 weeks in photoperiodic conditions, one group of hamsters was subjected to indirect calorimetry (group 1), and another group underwent μPET/CT scanning after 18F-FDG administration (group 2). A third group (group 3) was used specifically for reproductive tissue mass measures (n = 12/ group; 72 total) and pelage scoring. Pelage scores were assigned in group 3 hamsters using the four-point scale: summer pelage = 1, winter pelage = 4 [9,10]. This study was conducted under approval of The Ohio State University Institutional Animal Care Committee and all procedures followed the National Institutes of Health Guide for the Use and Care of Laboratory Animals and international ethical standards [11].
Indirect calorimetry
The comprehensive laboratory animal monitoring system (CLAMS) (Oxymax, Columbus Instruments, Columbus, OH, USA) is an integrated indirect calorimetry system, capable of simultaneously monitoring oxygen consumption (VO2), CO2 production (VCO2), and physical activity. Oxymax software allows accurate measurement of energy expenditure (heat production) and respiratory exchange ratio (RER), a measure of carbohydrate (RER = 1.0) versus fat (RER approaching 0.7) relative oxidation, independent of protein oxidation. All these parameters taken together provide an index of living cost of the animal known as “metabolic rate.”
Following a ~20 h fast to minimize the influence of previous food intake on metabolic measures, hamsters were separated, and placed into individual metabolic chambers (10.4 × 8.6 × 20.2 cm) for the duration of the test (24 h) with ad libitum food and water access. Although social isolation during CLAMS measurements may have caused a stress response, all hamsters underwent this manipulation. A previous study has demonstrated that separation-induced elevations in cortisol are not evident until at least 4 weeks after separation [12]. Photoperiodic conditions were maintained throughout the duration of the test. Volume O2 (mL/h), volume CO2 (mL/h), respiratory exchange ratio (RER), heat (kcal/h), and physical activity (x and z axis infra-red beam breaks) were assessed every 18 min for each animal over the 24 h period (resulting in ~80 sampling points for each animal). Animal masses were determined immediately preceding the test, and this mass value was used as a covariate in subsequent analyses. Previous analysis of data collected from Siberian hamsters in the CLAMS apparatus has shown that the initial 16 h of testing does not differ from subsequent recording days, indicating that a ‘habituation’ period is likely not required for this species [5]. The following equations were used:
Respiratory Exchange Ratio (RER) = Volume CO2/Volume O2
Calorific Value (CV) = 3.815 + 1.232 x RER
Heat (energy expenditure) = CV x VO2
Constants for CV calculation were derived from previous data on oxidation of mixtures of carbohydrate and fat [13]. Kleiber’s law or similar exponent modifications were not used as these data modifications have been proven to be inappropriate and provide inaccurate results [14].
Micro-positron emission tomography (μPET) and biodistribution of 18F-FDG
To determine organ specific metabolic activity between photoperiodic conditions, hamsters in group 2 were fasted overnight and then received a 500 μCi intra-peritoneal injection of the glucose analogue 2-Deoxy-2-[18F] fluoro-Dglucose (18F-FDG) (110 min half-life) the following morning. After 70 min, a subset of 4 hamsters from each photoperiodic group was individually anesthetized with isoflurane (5% induction, 1.5% maintenance), and had continuous PET/CT (Inveon, Siemens Preclinical, Knoxville, TN) images taken for 15 min (10 min PET, 5 min CT). Hamsters were then euthanized by isoflurane overdose/decapitation 90 min post-injection. This time point was used as we determined the peak temporal distribution in a pilot study using hamsters not included in any subsequent analyses. Biodistribution (BioD) of 18F-FDG uptake (12 hamsters per group) was determined for blood, lungs, heart, liver, spleen, pancreas, whole GI, kidneys, muscle (biceps femoris), skin, brain, and whole remaining carcass via a Wizard II gamma counter (Perkin Elmer, Waltham, MA, USA). To examine gross changes in organ metabolism, BioD data were analyzed at the whole organ/tissue level (%ID/organ).
Then, values were corrected for organ mass (%ID/gram) to examine per-gram glucose metabolic efficiency. Data are displayed as percentage injected dose (%ID):
%ID/organ = {(radioactivity recovered/organ)) / (administered dose of radioactivity)} × 100.
%ID/gram = {(radioactivity recovered/gram (of organ/ tissue))) / (administered dose of radioactivity)} × 100
To further investigate brain-specific glucose metabolism, an average correlation of CT units (-250) was used to determine a constant to differentiate bone from soft tissue. This number was used to generate a cerebral cavity volume (a proxy for brain volume) for each animal’s CT scan terminating at the base of the cerebellum. The volumes were individually investigated to remove artifacts. No smoothing was used. These volumes were used in Inveon Research Workplace (IRW) to assess the exact volume, density, and specific activity of the material contained within the Volume of interest (VOI) (i.e., the braincase). The estimated voxel resolution for reconstruction was 0.776 × 0.776 × 0.796 mm, creating a spatial resolution of ~0.78 mm3.
Statistical analyses
All statistical analyses were conducted using SPSS Statistics version 22 (IBM, Armonk, NY, USA) and GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Data points within each photoperiod group that differed from the group mean by ≥ 2 standard deviations were excluded from analyses. Removal of outliers from BioD data altered the significance of our results. Because this was our first study using IP injection of 18F-FDG, we attribute the aberrant values to inappropriate injections. To determine differences within the 24-hour CLAMS sampling period, repeated-measures analysis of variance (ANOVA) was performed with sampling interval as the withinsubjects variable and photoperiod as the between-subjects variable, with body mass as a covariate [5,15]. Resultant means for each sampling interval were transferred into GraphPad with corresponding standard error of the mean values for visualization. If significant F-values were detected, post-hoc multivariate ANOVAs with body mass as a covariate were used for specific time-point comparisons. For comparison of mean values of glucose utilization between photoperiodic groups, general linear model multivariate ANOVA was used. If a group displayed unequal variance, then non-parametric tests were used (i.e., Mann-Whitney U). Mean differences with p values ≤ 0.05 were considered statistically significant.
Results
Effects of photoperiod on pelage, body and organ masses
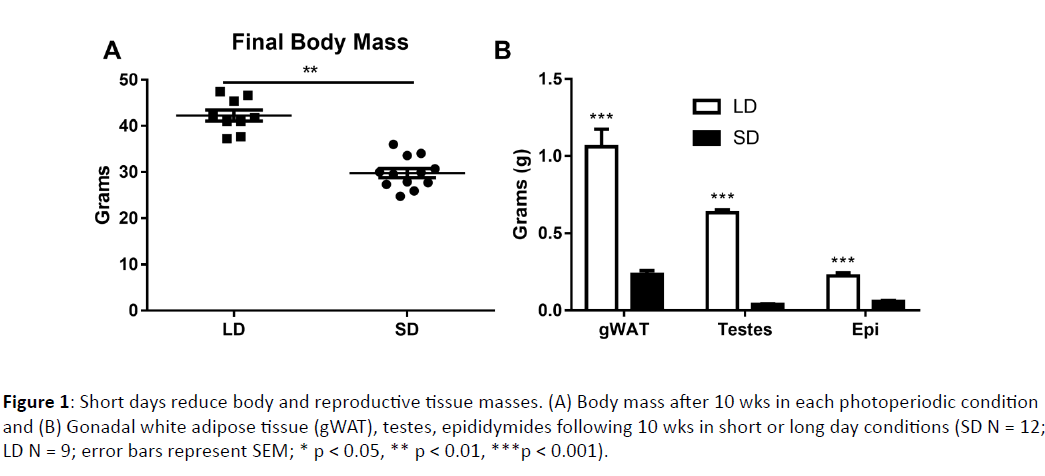
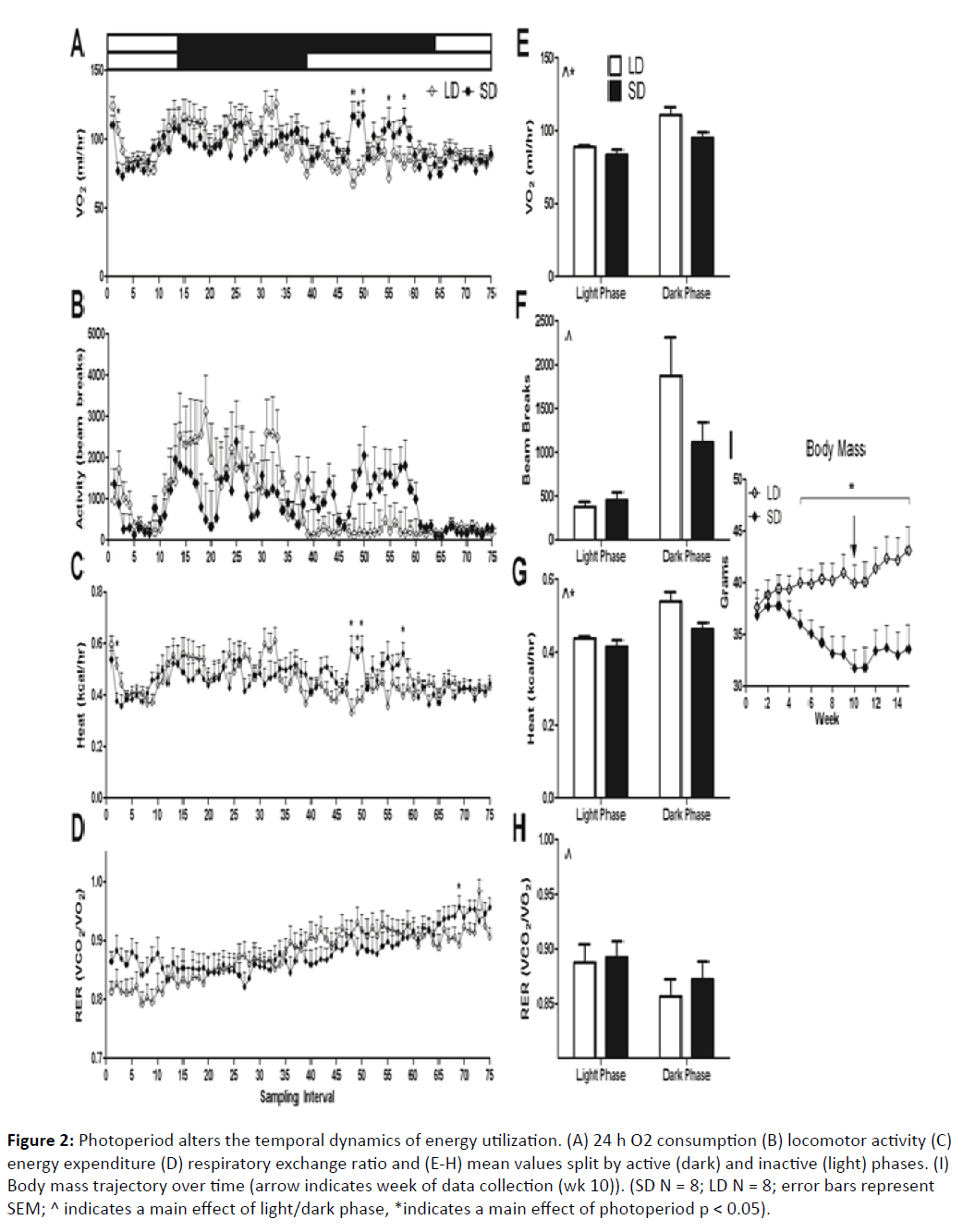
Body mass changed significantly in response to short photoperiods (F1, 14 = 11.3, p < 0.001). By week 5, differences became statistically significant (F1, 21 = 4.82, p < 0.05). At week 10, when all tests were performed, short day animals reduced their body mass from initial values by ~14%, and weighed significantly less than their long day counterparts (F1,21 = 8.84, p < 0.01) (Figure 1A). After 15 weeks, short day hamsters began to regain weight, and at this point their mass was reduced from initial values by ~10% (Figure 2I). By 10 weeks in their respective photoperiod conditions, short day hamsters grew a winter pelage and were easily distinguishable from their long day counterparts (F1, 19 = 391.597, p < 0.0001).
Figure 1: Short days reduce body and reproductive tissue masses. (A) Body mass after 10 wks in each photoperiodic condition and (B) Gonadal white adipose tissue (gWAT), testes, epididymides following 10 wks in short or long day conditions (SD N = 12; LD N = 9; error bars represent SEM; * p < 0.05, ** p < 0.01, ***p < 0.001).
Figure 2: Photoperiod alters the temporal dynamics of energy utilization. (A) 24 h O2 consumption (B) locomotor activity (C) energy expenditure (D) respiratory exchange ratio and (E-H) mean values split by active (dark) and inactive (light) phases. (I) Body mass trajectory over time (arrow indicates week of data collection (wk 10)). (SD N = 8; LD N = 8; error bars represent SEM; ^ indicates a main effect of light/dark phase, *indicates a main effect of photoperiod p < 0.05).
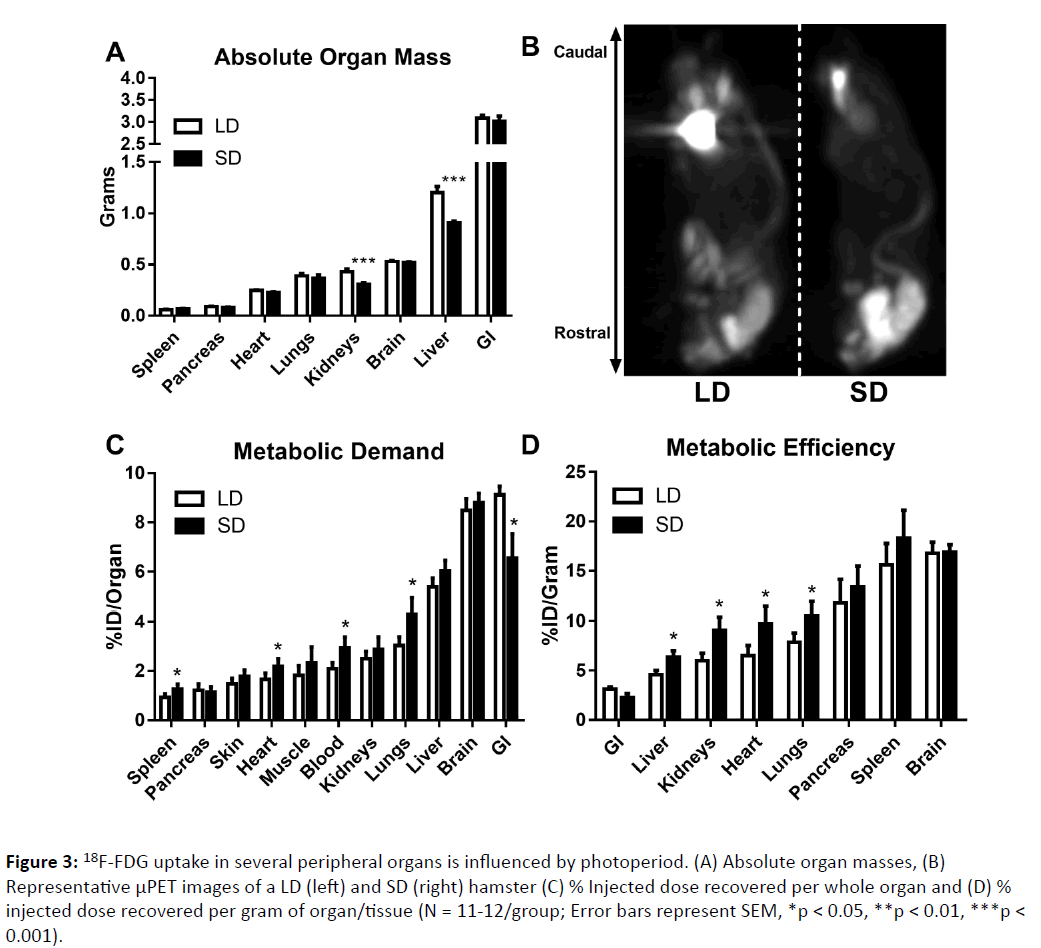
Reproductive tissue masses were obtained to further determine photoperiodic responsiveness. No short day exposed hamsters met criteria for non-responder status (via Zscore analyses). Long day animals had larger testes (F1, 19 = 1101.57, p < 0.0001) and epididymides (F1, 18 = 86.61, p < 0.0001) than short day hamsters. Short day hamsters did not have larger spleens than long day hamsters (F1, 19 = 0.740, p > 0.05), although this value was enhanced relative to body mass in short day animals (F1,19 = 7.122, p < 0.05). Long day adapted hamsters had larger gonadal white fat pad masses compared to short day hamsters (F1, 18 = 61.67, p < 0.0001) (Figure 1). Short photoperiods reduced the liver mass (F1, 22 = 22.23, p < 0.0005) and kidneys (F1, 22 = 19, p < 0.0003) as well, without similar changes in any of the other organs examined (p > 0.05 in all cases) (Figure 3A).
Figure 3: 18F-FDG uptake in several peripheral organs is influenced by photoperiod. (A) Absolute organ masses, (B) Representative μPET images of a LD (left) and SD (right) hamster (C) % Injected dose recovered per whole organ and (D) % injected dose recovered per gram of organ/tissue (N = 11-12/group; Error bars represent SEM, *p < 0.05, **p < 0.01, ***p < 0.001).
Effects of photoperiod on energy expenditure
Photoperiod influenced the temporal dynamics of VO2 consumption (F1,14 = 1.473, p < 0.01), respiratory exchange ratio (VCO2/VO2) (F1,14 = 2.68, p < 0.001), and heat production (F1,14 = 1.43, p < 0.05) when examined across the entire sampling period (~24h). Locomotor activity, as determined by infrared beam breaks, was not different between groups (F1,14 = 1.26, p > 0.05) (Figure 2B). To examine phase-specific differences in these parameters, light and dark phases were split and analyzed. Dark phase VO2 consumption was higher than light phase consumption (F1,14 = 51.07, p < 0.0001) and short day exposed mice showed a marginal reduction in VO2 consumption in both phases (F1,14 = 4.56, p = 0.05) (Figures 2A and 2E). Dark phase locomotor activity was higher in both photoperiodic conditions than in the light phase (F1,14 = 22.44, p < 0.001). Photoperiod did not influence light or dark phase locomotor activity (F1,14 = 1.58, p > 0.05). Dark phase heat production was increased (F1,14 = 41.25, p < 0.0001) compared to the light phase. Additionally, short day exposed mice reduced heat production in both phases compared to their long day counterparts (F1,14 = 4.5, p = 0.05) (Figures 2C and 2G). RER was increased during the light phase compared to the dark phase independent of photoperiod (F1,14 = 18.64, p < 0.001). No photoperiodic differences within either phase were detected in RER (F1,14 = 0.22, p > 0.05). Due to the differential lengths of the dark phases between groups, it is important to examine potential metabolic differences occurring specifically during the extended dark phase SD hamsters experience. Indeed, SD hamsters displayed increased VO2 consumption specifically during their late dark phase compared to LD hamsters (F1,14 = 5.16, p < 0.05). These results suggest that after 10 weeks exposure to short photoperiods, hamsters changed their basal metabolic profiles over the course of the day, and marginally reduced total energy expenditure. Without multiple-day indirect calorimetry data, typical analyses for differences in circadian rhythms are not possible.
Photoperiod influenced glucose metabolism in several peripheral organs (Figures 3B and 3C). Short day exposed hamsters displayed an increase in metabolic demand within the blood (F1,20 = 6.457, p < 0.05), lungs (F1,21 = 6.542, p < 0.05), heart (F1,20 = 6.966, p < 0.05) and spleen (F1,20 = 8.396, p < 0.05) compared to long day adapted hamsters. Short day hamsters simultaneously reduced glucose utilization in the lower GI (U = 101, p < 0.05). When metabolic efficiency was measured in each organ, short day exposed hamsters showed increased glucose utilization per unit mass in the lungs (F1,20 = 4.787, p < 0.05), heart (F1,20 = 4.422, p < 0.05), liver (F1,20 = 5.649, p < 0.05), and kidneys (F1,20 = 5.851, p < 0.05) compared to their long day counterparts. When analyzing μPET/CT images (4 per group), the braincase 18F-FDG standard uptake value (SUV) did not differ between groups (p > 0.05), indicating that photoperiod did not alter whole brain glucose metabolism (Fig. 4), although small sample sizes for this measure (4/group) and/or the limits of spatial resolution might explain this discrepancy.
Discussion
Our results provide evidence that photoperiod alters organspecific glucose metabolism in adult male Siberian hamsters. Similar to other small mammals, the most metabolically active organs (highest 18F-FDG uptake) in basal conditions were the liver, brain, and lower gastrointestinal system (Figure 3B). These organs differed significantly in their fuel efficiency; the brain was the most metabolically active organ per unit mass, whereas the lower GI system was the least in both photoperiodic conditions (Figure 3C). Significant differences were detected in glucose utilization between photoperiodic conditions. Short day hamsters increased metabolic demand in the spleen, heart, lungs, and blood. Simultaneously hamsters housed in short photoperiods decreased energy utilization by the lower gastrointestinal system, indicating a reallocation of energy use from the digestive system (or other organs not evaluated in this study) to other somatic organs in response to decreasing day lengths. Additionally, short day hamsters reorganized the metabolic efficiency of several organs, increasing the per unit mass energy usage of the liver, kidneys, heart, and lungs compared to their long day counterparts (Figure 3C). As these hamsters regressed their reproductive systems (Figure 1B), the increased metabolic demand in the aforementioned organs may reflect a reallocation of energy from reproductive tissues to be used for somatic maintenance or immune function [16].
Many previous studies demonstrate short-day enhancement of immune function in Siberian hamsters (e.g. [17,18]; and reviewed in [19]). Short-day enhancement of immune function is thought to counteract stress-induced immunosuppression during harsh winter conditions. We observed a relative increase in splenic metabolic demand (Figure 3B) in response to short days. The spleen is a secondary lymphoid organ that harbours a large reservoir of monocytes, ready to deploy upon immune stimulation [20], and thus plays a critical role in adaptive immunity. As proper immune function is critical for survival during the energetically demanding winter, a trade-off between the reproductive and immune systems reflects changes in energy allocation important for survival [16]. Our data are consistent with this hypothesis, and suggest that short-days increase the energetic demand of the spleen to putatively enhance immune defenses.
Short photoperiods promoted a catabolic state, as reflected in significantly reduced body and reproductive tissue masses over the course of 10 weeks (Figure 1). Previous studies have demonstrated that short photoperiods reduce both total energy expenditure and circadian organization in Siberian hamsters; these parameters are regained by subsequent housing in long photoperiod conditions [5]. We confirmed these previous findings, as short day hamsters displayed reduced heat production and VO2 consumption compared to long day hamsters (Figures 2E-2H). Multiple-day sampling may be required to maximally detect photoperiod-induced changes in basal metabolic rate. It is notable that photoperiod-induced differences in RER were not apparent given the large changes in gonadal WAT mass (Figure 1B). At week 10, hamsters maintained in short-day conditions reached the nadir of their body mass loss (Figure 2I)., which could indicate that WAT catabolism was likely limited at this point, as reflected in similar RER values between groups. Indeed, in a study by Warner and colleagues [5], RER decreased initially in response to short photoperiods, but steadily returned to values similar to those of long day adapted hamsters by ~12 wks.
Brown adipose tissue (BAT), when fully activated, can be the most metabolically active tissue in the body. Short dayadapted Siberian hamsters significantly increase brown adipose tissue activity [21], which could significantly modulate metabolic phenotype. However, near thermoneutrality [3,7], BAT is not significantly activated and likely contributes little to thermogenesis [22]. To observe further ecologically relevant metabolic differences between short- and long-day adapted hamsters, energy expenditure may need to be assayed in more naturalistic conditions where temperature fluctuations and food availability are manipulated. Alternatively, others have observed torpor behavior in the home cage [23] that is no longer evident when animals are housed in indirect calorimetry chambers [5], indicating that these measurements may alter normal metabolic responses to photoperiod.
The lack of differences in whole brain glucose metabolism is of special interest, as exposure to short photoperiods reduces cognitive and affective behavioral capacity in several photoperiodic rodents [2,24-27]. Data on seasonal plasticity in cognitive function in Siberian hamsters, however, is lacking. Reduced regional brain glucose metabolism has been previously associated with decreased cognitive and affective capacity, and learning and memory in humans and aged rodents (e.g. [28-31]). The lack of differences we observed in the present (Figure 3) study may suggest that affective changes observed in response to short photoperiods [32] are independent of whole brain glucose metabolism in this species. Alternatively, even without whole brain changes in metabolism, specific regional glucose metabolism may be significantly altered, or decreases in brain glucose utilization may only be evident during torpor behavior.
In sum, our data demonstrate that short-days induce a reorganization of metabolic demand and efficiency in tandem with reproductive regression. These changes likely reflect a trade-off in reproductive function for somatic maintenance and immune enhancement, important factors for survival. These results have broader implications for understanding seasonal control of metabolism, adult metabolic plasticity, and life-history trade-offs that occur in response to a discrete environmental variable (i.e., photoperiod).
Acknowledgments
The authors thank The Ohio State University Laboratory Animal Resources (ULAR) personnel for the excellent care they provided the animals used in this study. This work was supported by NSF IOS-1118792 to RJN.
References
- Bartness TJ, Hamilton JM, Wade GN, Goldman BD(1989) Regional differences in fat pad responses to short days in Siberian hamsters. Am J Physiol 257: R1533-R1540.
- Walton JC, Weil ZM, Nelson RJ (2011) Influence of photoperiod on hormones, behavior, and immune function. Front Neuroendocrinol 32: 303-319.
- Heldmaier G, Steinlechner S (1981) Seasonal control of energy requirements for thermoregulation in the Djungarian hamster (Phodopussungorus), living in natural photoperiod. J Comp Physiol B 142: 429-437.
- Nelson RJ (1987) Photoperiod-nonresposive morphs: A possible variable in microtine population-density fluctuations. The American Naturalist 130: 350-369.
- Warner A, Jethwa PH, Wyse CA, I’Anson H, Brameld JM, et al. (2010) Effects of photoperiod on daily locomotor activity, energy expenditure, and feeding behavior in a seasonal mammal. Am J Physiol 298: R1409-R1416.
- Bender DA (2008) Introduction to Nutrition and Metabolism. (4th edn.). Boca Raton, FL: CRC Press.
- Kauffman AS, Cabrera A, Zucker I (2001) Energy intake and fur in summer- and winter-acclimated Siberian hamsters (Phodopussungorus). Am J Physiol 281: R519-R527.
- Jefimow M, Wojciechowski M, Masuda A, Oishi T (2004) Correlation between torpor frequency and capacity for non-shivering themogenesis in the Siberian hamster (Phodopussungorus). J ThermBiol 29: 467-641.
- Duncan MJ, Goldman BD (1984) Hormonal regulation of the annual pelatecolor cycle in the Djungarian hamster, Phodopussungorus. II. Role of prolactin. J ExpZool 230: 97-103.
- Butler MP, Zucker I (2009) Seasonal pelage changes are synchronized by simulated natural photoperiods in Siberian hamsters (Phodopussungorus). J ExpZool A Ecol Genet Physiol 311A: 475-482.
- Portaluppi F, Smolensky MH, Touitou Y (2010) Ethics and methods for biological rhythm research on animals and human beings. ChronobiolInt 27: 1911-1929.
- Castro WL, Matt KS (1997) Neuroendocrine correlates of separation stress in the Siberian dwarf hamster (Phodopussungorus). PhysiolBehav 61: 477-484.
- Lusk G (1928) The science of nutrition. Philidelphia, PA, WB Saunders Co.
- Tschöp MH, Speakman JR, Arch JR, Auwerx J, Brüning JC, et al. (2011) A guide to analysis of mouse energy metabolism. Nat Methods 9: 57-63.
- Arch JR, Hislop D, Wang SJ, Speakman JR (2006) Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 30: 1322-1331.
- Nelson RJ, Demas GE (1996) Seasonal changes in immune function. Rev Biol 71: 511-548.
- Wen JC, Dhabhar FS, Prendergast BJ (2007) Pineal-dependent and -independent effects of photoperiod on immune function in Siberian hamsters (Phodopussungorus). HormBehav 51: 31-39.
- Pawlak J, Golab M, Markowska M, Majewski P, Skwarlo-Sonta K (2009) Photoperiod-related changes in hormonal and immune status of male Siberian hamsters, Phodopussungorus. Comp BiochemPhysiol A MolIntegrPhysiol 152: 299-303.
- Weil ZM, Borniger JC, Cisse YM, AbiSalloum BA, Nelson RJ (2015) Neuroendocrine control of photoperiodic changes in immune function. Front Neuroendocrinol 37: 108-118.
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, et al. (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325: 612-616.
- Demas GE, Bowers RR, Bartness TJ, Gettys TW (2002) Photoperiodic regulation of gene expression in brown and white adipose tissue of Siberian hamsters (Phodopussungorus). Am J Physiol 282: R114-R121.
- Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277-359.
- Murphy M, Jethwa PH, Warner A, Barrett P, Nilaweera KN, et al. (2012) Effects of manipulating hypothalamic tri-iodothyronine concentrations on seasonal body weight and torpor cycles in Siberian hamsters. Endocrinology 153: 101-112.
- Workman JL, Nelson RJ (2011) Potential animal models of seasonal affective disorder. NeurosciBiobehav Rev 35: 669-679.
- Workman JL, Manny N, Walton JC, Nelson RJ (2011) Short day lengths alter stress and depressive-like responses, and hippocampal morphology in Siberian hamsters. HormBehav 60: 520-528.
- Pyter LM, Reader BF, Nelson RJ (2005) Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscusleucopus). J Neurosci 25: 4521-4526.
- Walton JC, Chen Z, Travers JB, Nelson RJ (2013) Exogenous melatonin reproduces the effects of short day lengths on hippocampal function in male white-footed mice, Peromyscusleucopus. Neuroscience 248: 403-413.
- Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, et al. (2009) FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging 36: 811-922.
- Gage FH, Kelly PA, Björklund A (1984) Regional changes in brain glucose metabolism reflect cognitive impairments in aged rats. J Neurosci 4: 2856-2865.
- Arnaiz E, Jelic V, Almksvist O, Wahlund LO (2001) Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. Neuroreport 12: 851-855.
- Videbech P (2000) PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. ActaPsychiatrScand 101: 11-20.
- Prendergast BJ, Nelson RJ (2005) Affective responses to changes in day length in Siberian hamsters (Phodopussungorus). Psychoneuroendocrinology 30: 438-452.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences