How to deal with parathyroid carcinoma: case report, histological difficulties and literature review
Anass Haloui, Mouhoub Mohammed, Achraf Miry, Jabi rachid, Bouziane Mohammed, Amal Bennani
DOI10.36648/2572-5432.6.1.32
1Pathology Department, Mohamed VI University Hospital, Oujda, Morocco
2Department of General Surgery, Mohammed VI University Hospital, Faculty of Medicine and Pharmacy, Oujda, Morocco
- Corresponding Author:
- Anass Haloui
Pathology Department
Mohamed VI University Hospital
Oujda, Morocco
Tel: 212678026455
E-mail: halouianass@gmail.com
Received Date: November 17, 2020; Accepted Date: December 11, 2020; Published Date: December 18, 2020
Citation: Haloui A, Mohammed M, Miry A, Rachid J, Mohammed B, et al. How to deal with parathyroid carcinoma: case report, histological difficulties and literature review. J Clin Mol Endocrinol. 2020, 6:1.32. doi: 10.36648/2572-5432.6.1.32
Copyright: © 2020 Mbamalu ON, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The histological diagnosis of parathyroid carcinoma is often difficult due to the existence of many potentially misleading similarities, between this malignant entity and its main differential diagnosis: parathyroid adenoma.
Several histological criteria of malignancy have been proposed in order to solve this diagnosis problem: the presence of fibrous bands emerging from a thick capsule dividing the tumor proliferation into lobules, tumor cells arranged in clusters and trabeculae, moderate to clear cytonuclear atypia and low mitotic activity. Unfortunately, these criteria are not exclusive to carcinomas and can also be seen in cases of adenomas.
The more relevant histological criteria of malignancy are proposed, such as atypical mitosis, capsular invasion, vascular invasion or the exceptional perineural invasion. But these criteria are rarely found. Infiltration of the thyroid gland, adjacent soft tissue and the occurrence of metastases remain the only indisputable signs of malignancy.
Immunohistochemistry can contribute to the differential diagnosis, especially with the loss of expression of Parafibromin, commonly found in carcinomas unlike adenomas, or the expression of parathormone, allowing the elimination of a thyroid tumor.
Keywords
Parathyroid carcinoma; Parathyroid adenoma; Histological difficulties; Comparative table
Introduction
Parathyroid carcinoma (PC) is a rare endocrine malignancy that remains enigmatic. Commonly a sporadic disease, it may occur in familial PHPT, namely the hyperparathyroidism-jaw tumor syndrome (HPT-JT), and very rarely, in the multiple endocrine neoplasia type1 (MEN1) [1]. Usual clinical features are mainly due to the excessive secretion of Parathormone (PTH) causing hypercalcemia, hypophosphatemia, and hypercalciuria. Thus, the clinical phenotype is characterized by symptoms of hypercalcemia and end-organ damage, including renal failure, bone disease, cardiac arrhythmia and neurocognitive dysfunction [2].
The disease commonly has an indolent and slow progressive course, and most patients ultimately succumb to complications of relentless hypercalcaemia rather than tumour invasion or metastatic spread. None of these, however, are strict rules. Indeed, the very first documented case of parathyroid carcinoma was a non-functioning carcinoma reported by Fritz De Quervain, in 1904 [3]. It wasn’t until 1933 that Sainton and Millot were first to report a patient with a functioning parathyroid carcinoma [4]. The main differential diagnosis is the parathyroid adenoma, which shares with parathyroid carcinoma a lot of clinical, biological and histological features that can make the diagnosis challenging.
Given the lack of specific clinical and biological features, the distinction between benign and malignant parathyroid tumor is often difficult preoperatively and very often it is diagnosed postoperatively at histological examination. However, even histology of PC can be equivocal or frankly misleading. Thus, it is common that the diagnosis of PC is made a posteriori, when local recurrence or distant metastases occur [5]. Although no breakthrough have been made regarding curative options, the greater expansion of the parathyroid carcinoma’s molecular pathogenesis knowledge, has led to the development of diagnostic markers that can be helpful in making the diagnosis more certain, particularly when the histological presentation is ambiguous.
Surgical resection is the accepted ‘gold standard’. There is now a growing consensus on the use of adjuvant radiotherapy as it has been shown to provide a survival benefit [6]. The understanding of the natural history and prognostic factors of the parathyroid carcinoma was for a long time restrained by the rarity of this kind of neoplasia on the one hand, and on the another hand by the paucity of series and case reports in the literature, preventing a clear consensus about its surgical and adjuvant treatment. The aim of this article is to discuss histological criteria’s of this tumor, and to make a review of the literature in order to clarify it pathogenesis, clinical features, or pathology diagnosis, and on the basis of this, to shed the light on the management of this condition, for practitioners generally, and for pathologists specifically.
Case Report
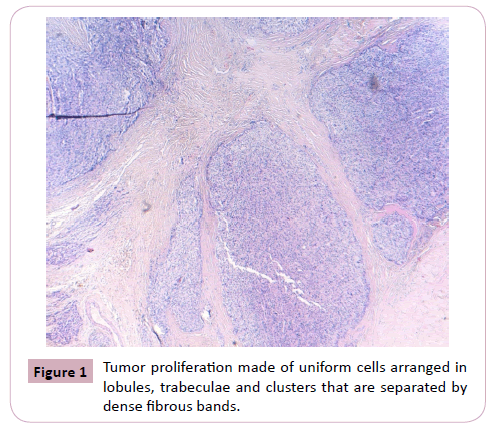
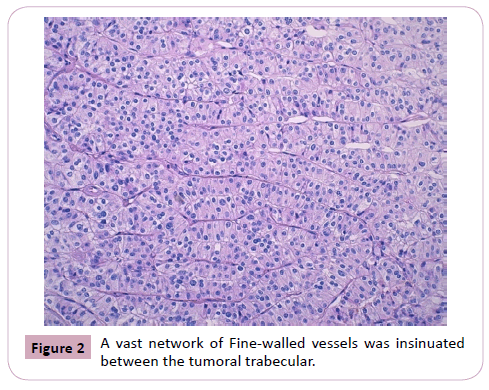
We report the case of a 54-year-old man, who consulted in January 2019 and for whom the laboratory work-up revealed hypercalcemia and elevated serum parathormone. CT scan revealed a mass of the right inferior parathyroid gland, with images of liver metastasis. Right parathyroidectomy was performed. Macroscopically, the parathyroid mass was badly limited, of firm consistency, measuring 3 x 2 x 1.5 cm with a weight of 6.9 grams. On section, it comprised multiple whitish color nodules, of variable sizes, with fibrous and hemorrhagic features. Histological examination revealed a badly limited tumor proliferation, made of uniform cells arranged in lobules, trabeculae and clusters that are separated by dense fibrous bands. A vast network of finewalled vessels was insinuated between the tumoral trabeculae. (Figure 1 and 2)
Neoplasic cells were roughly polygonal. Numerous cytonuclear atypias were observed, with irregular, nucleated nuclei and an abundant eosinophilic, sometimes clear cytoplasm. Mitotic activity was estimated at 7 Mitosis per 10 high magnification fields (Figure 3).
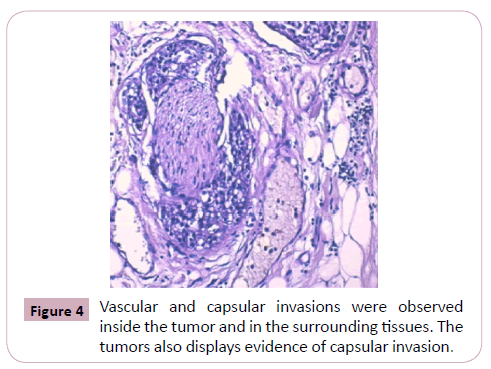
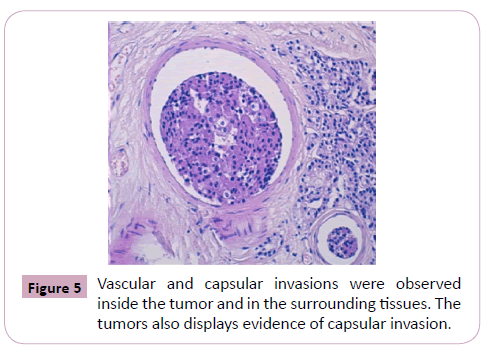
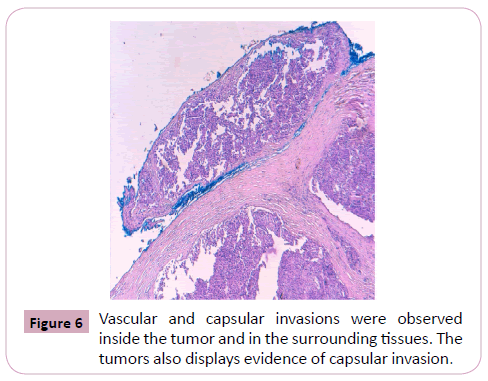
Vascular and capsular invasions were observed inside the tumor and in the surrounding tissues.
The tumors also displayed evidence of capsular invasion (Figure 4, 5 and 6).
However, some tumor cells that exceeded the peripheral celluloadipose tissue were observed, without true invasion of adjacent thyroid tissue. The diagnosis of (PC) was based on the criteria of invasion and local aggressiveness, and the presence of hepatic metastasis.
Discussion
Epidemiology
Parathyroid cancer is a rare entity, with an incidence of 0.005% of all registered cases in the The National Cancer Registry Database in the USA [7]. It accounts for 1% of all cases of primary hyperparathyroidism in the United States and most Western European countries [5]. Most cases of parathyroid carcinoma are diagnosed between the ages of 44 and 54 years, which is approximately a decade earlier than the median age of patients with parathyroid adenomas [5,8,9]. It occurs rarely in children, although patients as young as 8 years of age have been reported [10]. In contrast to parathyroid adenomas where women predominate over man by a ratio of 3-4:1, the sex distribution is equal for parathyroid carcinomas [11].
EtiologyThe etiology of parathyroid carcinoma remains unknown. No predisposing dietary factors have been identified [12]. Inadequate sunlight exposure has been observed to be a risk factor for benign hyperparathyroidism but not for carcinomas, and the potential role for prior neck irradiation is less clear than in PA [1]. Rare cases of PC have been reported in patients with end-stage renal disease [13]. Commonly a sporadic disease, PC has also been reported in familial isolated hyperparathyroidism (FIHP) and, rarely in multiple endocrine neoplasia type 1, MEN1 and MEN2A syndrome [14-17].
PathogenesisEven though the cause of PC remains unclear, a few revealing associations exists between some chromosomal aberrations and the pathogenesis of PC. Kytola et al. reported that losses of 1p, 4q and 13q, and gains of 1q, 9q, 16p, 19p and Xq, were more commonly observed in parathyroid carcinoma than in adenomas, whereas the main chromosomal aberration observed in parathyroid adenoma, which is the loss of 11q13 region, is absent in carcinoma. [18]. Several different mutations have also been implicated in the ontogenesis process of parathyroid carcinoma, including the retinoblastoma (Rb), p53, breast carcinoma susceptibility (BRCA2) and cyclin Dl/parathyroid adenomatosis gene 1 (PRAD1) genes. But none of these have been assigned a primary role in pathogenesis [19-24].
The hyperparathyroidism jaw tumor (HPT-JT) has provided the best evidence for a defined gene in parathyroid cancer. The responsible gene is known now as HRPT2 [25]. It codes for a nuclear protein named “Parafibromin” [26,28], which acts as a regulator of transcription. Over expression of this protein causes inhibition of cell proliferation and G1 phase arrest [29,30]. Non-functional isoforms, such as mutations in hereditary hyperparathyroidism jaw tumor syndrome, have anti-apoptotic effects.
Mutation of HRPT2 was found to be much more commonly present, in cases of sporadic parathyroid carcinoma than in adenomas (up to 76% of carcinomas vs 0.8e1.8% of adenomas) [31,32]. This evidence suggest the significant role, the HRPT2’s mutation have in the pathogenesis of parathyroid carcinoma. Furthermore, in order to facilitate early diagnosis and eventual cure for the disease, monitoring on a regular basis, with serum calcium estimation, for individuals with HRPT2 mutation has been proposed. [33]. However, more studies and a further apprehension of PC oncogenesis will certainly be helpful to promote the early diagnosis and provide new biological targets for more efficient therapy.
DiagnosisA) Clinical Features
Usual clinical features of patients with functioning parathyroid carcinoma are mainly due to the excessive secretion of Parathormone (PTH). Thus, the clinical presentation is characterized by symptoms of moderate to severe hypercalcemia, associated with renal and bone disease, cardiac arrhythmia and neurocognitive dysfunction [34]. Physical examination is commonly unrevealing, but the finding of palpable cervical mass and laryngeal nerve palsy may predict the presence of PC [2]. Suspicion of PC on a clinical level is determining, for it may guide the extent of the initial surgery which is crucial for definitive cure. Rarely parathyroid carcinomas may be non-functional [35].
B) Biochemical Features
As might be expected, parathormone levels are markedly elevated in patients with carcinoma²s (3-10 times above the upper normal limit) contrary to those with adenomas in whom parathormone levels are typically more modest [2]. Most patients with adenomas have serum calcium levels within 1 mg/ dL above the normal limit, whereas the levels in patients with parathyroid carcinoma are usually in excess of 14 mg/dL [5]. Hypophosphatemia, and hypercalciuria could also be found. PC should be suspected in patients with acute hyperparathyroidism [2]. The combined finding of markedly elevated serum calcium (>12 mg/dl (>3 mmol/l)) and a large parathyroid lesion (>3 cm) (the so-called >3 + >3 rule should raise the suspicion of PC) [36].
C) Imaging Features
Various imaging modalities such as ultrasonography, Tc99mlabeled sestamibi scintigraphy (MIBI), computed tomography (CT), and magnetic resonance imaging (MRI) can be used to investigate parathyroid carcinoma. These investigations are not diagnostic but are effective in determining the size and location of the abnormal parathyroid gland, which is of value in planning curative resection. The choice of imaging should be guided by the clinical presentation. Neck ultrasound and MIBI help to localize the abnormal parathyroid tissue prior to surgery [37]. At neck ultrasound, a size >3 cm, a lobulated non-homogeneous pattern, marked hypoechogenicity, degenerative changes, calcifications, and irregular halo sign may raise the suspicion of PC [38]. MIBI allows identifying eutopic and ectopic parathyroid tissue as well as recurrent disease, but there are no specific features to distinguish benign and malignant tumors. Both CT and MRI can accurately localize the primary tumor or its recurrence, their relationship with adjacent structures and lymph node metastases [2].
D) Intraoperative suspicion
The treatment of parathyroid carcinoma is essentially surgical. Given the lack of specific preoperative diagnostic tools, most parathyroid carcinomas are detected incidentally and postoperatively, during the routine examination of surgical specimens [39]. In some cases, patients initially undergo operation for presumed benign primary hyperparathyroidism, which turned out to be malignant in the postoperative assessment.
Thus, for patient with an unanticipated diagnosis of parathyroid carcinoma, re-operation may be warranted for disease control [39]. In fact, it is reported that 25% of cases of Parathyroid carcinoma are not recognized by the surgeon at the time of initial parathyroidectomy [40]. During surgery the presence of a firm to hard, greyish-white, lobulated mass surrounded by a dense fibrous capsule, with large size (> 3 cm if solid), should raise the suspicion that the lesion is malignant. In addition, infiltration into adjacent structures and the presence of enlarged lymph nodes is highly suggestive of parathyroid carcinoma. Frozen-section analysis is of little value, as the distinction between adenoma and well differentiated carcinoma is very difficult [41].
E) Histopathology
1) Macroscopic Features
The diagnosis of Parathyroid carcinoma is often challenging, given the fact that a lot of similarities exists between this malignant entity and the benign parathyroid adenoma. However, some differences in the macroscopic evaluation of the specimen received for histological examination, may suggest the malignancy (Table 1). Parathyroid carcinomas are usually solitary and arise from a single gland. However, there have been reports of multiglandular involvement [42]. Adenomas are also solitary tumors, although some cases of multiple sites have been reported.
| Parathyroid carcinoma | Parathyroid adenoma | |
| Number | Solitary , rarely multiple | Solitary, rarely multiple |
| Size | Average of 3cm diameter | Variable sizes |
| Weight | Average of 6.7g | Variable, 0.1g to 100g |
| Form | Irregular | Round to oval, ellipsoid |
| Encapsulation | May be encapsulated , which makes it indistinguishable from adenomas | Well encapsulated |
| Cross section | Irregular , poorly limited , lobulated Fibrous and hemorrhagic features possible Areas of necrosis may be apparent as soft yellow foci | Homogeneous, well circumscribed
Cystic and hemorrhagic rearrangements possible |
| Color | grayish-to-white | reddish brown |
| Consistency | Firm- hard | Soft |
| Infiltration | May infiltrate the thyroid, adjacent muscles, recurrent laryngeal nerve, trachea or oesophagus | Absent |
Table 1: Comparative table between the macroscopic features of parathyroid carcinoma and adenoma.
Both right and left inferior glands have been counted as the most common location. Ectopic tumors usually arise from glands in the mediastinum, but ectopic location does not increase the chance of malignant transformation of a gland [9, 43, 44]. In the series of Koea and Shaw, within 155 cases, seventy four cases (48%) occurred in the left inferior gland, although other series have noted a right inferior preponderance. The remaining carcinomas were evenly distributed between the left upper position (20 cases), right lower position (38 cases), and the right upper position (18 cases). Five carcinomas occurred in the mediastinum [45].
Parathyroid carcinoma most often appears as a poorly circumscribed mass, quite large with an average diameter over 3 cm, that may be palpable at presentation [31], and can weigh between 2 and 10 g. (overview rose). In the series of Wang and Gaz, 40 carcinomas ranged in size from 1.5 to 6.0 cm (average of 3 cm) with weights ranging from 1.5 to 27 g (average of 6.7 g) [9]. Adenomas on the other hand, are usually well limited, with variable sizes and their weight can reach up to 100 g. Occasional carcinomas may be grossly encapsulated and may resemble parathyroid adenomas.
On cross-section, parathyroid carcinomas are irregular, lobulated, with firm to hard consistency, and have a grayish-to-white color [45]. Occasional areas of necrosis may be apparent as soft yellow foci (over view noir 2005). While adenomas have a round-oval, or ellipsoid form, homogenous with a soft consistency and a reddish brown to tan in appearance [46]. Cystic and hemorrhagic features may be found.
Carcinomas are densely adherent to the adjacent soft tissues of the neck or the thyroid gland. It can infiltrate the ipsilateral lobe of the thyroid, the strap muscles and even the recurrent laryngeal nerve, trachea or esophagus [9]. Cases of synchronous parathyroid carcinoma and adenoma have been reported [47]. In our patient, parathyroid carcinoma was suspected macroscopically because of the badly limited character of the mass, with 3 cm diameter and a weight of 6.9 grams, and the finding of variable sizes, whitish color nodules on the cross section, associated with fibrous and hemorrhagic features.
2) Microscopic Features
Histopathological diagnosis of parathyroid carcinoma can be obvious in some cases, but others can be extremely difficult for the reason that plenty of features overlap with those of parathyroid adenoma. Indeed, in a large series of patients with metastases, up to 50% of them were initially classified as beginning tumors. [48]. several criteria’s should be considered.
Growth pattern
The growth pattern varies. Typically, a parathyroid carcinoma shows a thick fibrous capsule from which, thick acellular fibrous bands appear to extend into the central regions of the tumor and tend to divide it into sharply outlined, irregular shaped and sized compartments, creating a lobular appearance [49]. Most carcinomas display a solid growth pattern with tumor cells arranged in diffuse masses, small nests, or trabecular [50]. Sometimes, palisading or rosette-like growth may be present [49]. Other tumors may demonstrate follicular or spindle cell patterns, with rare tumors having a predominantly papillary growth pattern. Exceptionally, tumors may have a carcinosarcomatous pattern [50].
Tumor cells
Chief cells are almost always the predominant cell type. Sparse oxyphil and transitional oxyphil cells may be found and, very rarely, they may predominate [46]. Many carcinomas show mild to moderate variation in nuclear size and shape; however, occasional tumors show marked degrees of pleomorphism with coarsely granular chromatin and macronucleoli. The cytoplasm may vary from clear to faintly eosinophilic, and occasional tumors may be composed of oncocytic cells exclusively. Tumors composed of an Admixture of chief cells and oncocytes are relatively common [49]. Some tumors may show necrosis, a feature that should alert the pathologist to the possibility of carcinoma. [49]. This feature should be distinguished from the atypia encountered in parathyroid adenomas and other benign endocrine tumors.
Mitosis
The mitotic activity in carcinoma is generally low and common; however, it is also detected in PA [2]. A higher mitotic activity has been observed in poorly differentiated tumors and is associated with a poor prognosis [51]. Atypical mitosis usually indicates malignancy
Capsular invasion, vascular invasion and perineural invasion
The most specific features are capsular invasion vascular invasion, perineural invasion, or direct extension into adjacent soft tissues, but these can still be subjective to assess [52]. Invasion of the capsule is rather common. Less frequently vascular invasion also occurs in 10–15% of cases [53]. Whereas perineural invasion is exceptional.
3) Histological Difficulties Usually Encountered
In 1973, Schantz and Castleman [54] were the first to set criteria of malignancy, which can help to differentiate parathyroid carcinoma from adenoma, including:
• Lobular architectures separated with thick fibrous bands.
• Mitotic figures
• Capsular invasion
• Vascular invasion.
However, these findings are inconstantly observed in cases of PC and may also be observed in parathyroid adenomas [55]. Therefore, the lack of specific criterion of malignancy can be source of challenging histological difficulties, encountered in many levels of the histological examination. According to the World Health Organization, no specific has yet been identified.
Growth patternFibrous bands were present in 90% of the case studied by Schantz and Castleman. Such fibrosis is not specific though, and may also be seen in large adenomas that have undergone degenerative changes [52]. Bondeson et al. [55] have noted that as many as 1/5 of carcinomas do not show the typical fibrosis and lobular growth pattern; these features can also be seen in parathyroid hyperplasia and adenomas.
Tumor cells atypiaThe epithelial cells of a carcinoma are larger than normal chief cells, with round to oval nuclei; the cytoplasm may be clear or oncocytic. The nuclei are usually quite monotonous with pleomorphic nuclei being more typical of an adenoma [49, 54]. McKeown et al. [56] have pointed out that cellular pleomorphism and atypia are not reliable indicators of malignancy in endocrine tumors. Sometimes, however, a carcinoma may show marked nuclear pleomorphism with coarsely clumped chromatin and prominent nucleoli, distributed diffusely in contrast to the patchy pleomorphism of adenoma nuclei [57].
Mitosis
The mitotic activity in carcinoma is generally low and common; however, it is also detected in PA [2]. A higher mitotic activity has been observed in poorly differentiated tumors and is associated with a poor prognosis [51]. Atypical mitosis usually indicates malignancy
Capsular invasion, vascular invasion and perineural invasion
The most specific features are capsular invasion vascular invasion, perineural invasion, or direct extension into adjacent soft tissues, but these can still be subjective to assess [52]. Invasion of the capsule is rather common. Less frequently vascular invasion also occurs in 10–15% of cases [53]. Whereas perineural invasion is exceptional.
3) Histological Difficulties Usually Encountered
In 1973, Schantz and Castleman [54] were the first to set criteria of malignancy, which can help to differentiate parathyroid carcinoma from adenoma, including:
• Lobular architectures separated with thick fibrous bands.
• Mitotic figures
• Capsular invasion
• Vascular invasion.
However, these findings are inconstantly observed in cases of PC and may also be observed in parathyroid adenomas [55]. Therefore, the lack of specific criterion of malignancy can be source of challenging histological difficulties, encountered in many levels of the histological examination. According to the World Health Organization, no specific has yet been identified.
Growth pattern
Fibrous bands were present in 90% of the case studied by Schantz and Castleman. Such fibrosis is not specific though, and may also be seen in large adenomas that have undergone degenerative changes [52]. Bondeson et al. [55] have noted that as many as 1/5 of carcinomas do not show the typical fibrosis and lobular growth pattern; these features can also be seen in parathyroid hyperplasia and adenomas.
Tumor cells atypia
The epithelial cells of a carcinoma are larger than normal chief cells, with round to oval nuclei; the cytoplasm may be clear or oncocytic. The nuclei are usually quite monotonous with pleomorphic nuclei being more typical of an adenoma [49, 54]. McKeown et al. [56] have pointed out that cellular pleomorphism and atypia are not reliable indicators of malignancy in endocrine tumors. Sometimes, however, a carcinoma may show marked nuclear pleomorphism with coarsely clumped chromatin and prominent nucleoli, distributed diffusely in contrast to the patchy pleomorphism of adenoma nuclei [57].
Mitosis
Although mitotic activity is present in approximately 80% of parathyroid carcinomas, mitotic figures are also relatively common in adenomas and hyperplasia. Snover and Foucar [58] identified mitoses in 70% of adenomas, and similar findings have been reported by San Juan et al. [59]. And so, care must be taken by the pathologist in assessing mitotic activity in parenchymal cells, which have to be distinguished from mitoses in endothelial cells and other stromal elements. Atypical mitosis however, correlates more strongly with carcinoma [49, 52].
Capsular invasion
The capsules of carcinomas are generally thicker than those of adenomas of similar size [34]. True capsular invasion is highly predictive of malignancy and is said to be seen in 60% of cases. It is characterized by irregularly shaped and pointed tongue- like protrusions of parathyroid parenchyma through the capsule. This may be mimicked by entrapment of parenchymal cells within the capsule in adenomas [49, 52]. When assessing for capsular invasion, the pathologist should be wary of over-interpreting “pseudo invasion” of entrapped tumor cells, present within the capsule of adenomas, or by a multilobulated or multinodular growth pattern.
Vascular and perineural invasion
Vascular invasion is present in 10–15% of carcinomas [54]. The diagnosis of vascular invasion should be made only when tumor is present within capsular vessels or in vessels in the soft tissue surrounding the gland. To qualify as true invasion, tumor must be present within a vascular channel and must also be at least partially attached to its wall. This can easily be mimicked by artefactual separation of parathyroid epithelial cell groups from stroma when fresh specimens are handled for intraoperative frozen sections [60].
An endothelial covering may or may not be present. Perineural invasion is more objective to interpret but is relatively uncommon finding. In front of all histological difficulties that can stand in the way of parathyroid carcinoma diagnosis, Invasion of the thyroid and or/adjacent soft tissues, and metastases remains the only unequivocal features of parathyroid malignancy [61].
In our case, the neoplastic proliferation was poorly limited, arranged in lobules, trabeculae and clusters separated by dense fibrous bands. Numerous cytonuclear atypias were observed, with irregular, nucleolated nuclei and an abundant eosinophilic, sometimes clear cytoplasm. Mitotic activity was estimated at 7 Mitosis per 10 high magnification fields. Vascular and capsular invasions were observed inside the tumor and in the surrounding tissues, with evidence of capsular invasion. The diagnosis of (PC) was based on the criteria of invasion and local aggressiveness, and the presence of hepatic metastasis.
4) Immunochemistry
Immunohistochemistry may improve the diagnostic accuracy of parathyroid carcinoma. Parathyroid adenomas and carcinomas are generally positive for broad-spectrum cytokeratin’s. Additionally, they are positive for parathyroid hormone, chromogranin, and synaptophysin but are negative for thyroglobulin, calcitonin, and thyroid transcription factor-1 [62].
This immunohistochemical profile is of value in the distinction of parathyroid carcinomas from thyroid malignancies and other tumor types that may be present in this anatomic site. However, the distinction of benign and malignant parathyroid tumors is fraught with difficulty, and to date, limited data are available in equivocal cases. Increased labeling of cell cycle-associated proteins (Ki-67, cyclin D1) has been shown in parathyroid carcinoma as compared to adenoma, but overlap among these tumors has limited the utility of this approach [28,63].
Evaluation of HRPT2 gene abnormalities seems to be a more promising diagnostic tool [64]. Loss of heterozygosity or mutation at the HRPT2 gene and loss (total or focal) of parafibromin staining have been reported in the large majority of parathyroid carcinomas but very rarely in adenomas [28,64-68]. The retinoblastoma (RB) protein has been analyzed extensively in parathyroid tumors by immunohistochemistry. Cryns et al reported that parathyroid adenomas were consistently positive for the RB protein, whereas carcinomas were negative. Other studies, however, have been unable to demonstrate consistent differences between carcinomas and adenomas with respect to RB expression. [69, 70]
F) Differential Diagnosis
The main differential diagnosis is parathyroid adenoma, but parathyroid hyperplasia, thyroid cancer or, more rarely, metastasis of renal cell carcinoma, must also be excluded.
A) Parathyroid adenoma
This benign entity is much more common in women, whereas carcinoma occurs with equal sex ratio. Historically, the criteria for adenoma have generally included a pushing border with an absence of intralesional fibro adipose tissue, complete circumscription with a rim of ‘‘normal’’ parathyroid at the periphery, and an absence of lobular growth. The current definition is no longer purely histologic, but rather includes the effect of gland removal, with intraoperative PTH decrease and subsequent return to normocalcemia and long-term cure [71].
While most adenomas are composed of chief cells, a small percentage may be oxyphilic and rare ‘‘water-clear’’ adenomas have also been described. Lipoadenoma are another exceedingly uncommon entity, with fewer than 50 reported cases [71]. The Tables 1 and 2 sums up the main differences between PC and PA
| Parathyroid carcinoma | Parathyroid adenoma | |
| Growth pattern | • Thick fibrous capsule with fibrous bands dividing it into irregular compartments. • Mostly diffuse masses, lobules, nests, or trabeculae • Follicular, spindle cell patterns, papillary, palisading or rosette-like growth patterns may be present. |
• A single nodule, which is usually oval or bean-shaped and surrounded by a very thin fibrous capsule. • Nest ,trabeculae • Papillary and follicular pattern possible • rim of residual parathyroid tissue outside the capsule • Fibrous band may be present ( degenerative changes) |
| Tumor cells Atypia | • Mostly chief cells, rarely oxyphil cells • Mild to moderate atypia • Sometimes marked pleomorphism diffusely distributed |
• Predominantly either chief cells or oxyphil cells, sometimes both. Rarely water clear cells • Sometimes abundant fat cells: Lipoadenoma ( rare variant) • Often patchy nuclear pleomorphism |
| Mitosis | • Low mitotic activity common • Higher mitotic activity observed in poorly differentiated tumors and is associated with a poor prognosis • Atypical mitosis |
• May be present, should be of normal form. |
| Capsular invasion | • Common | • “pseudo invasion” of entrapped tumor cells within the capsule may mimic a true capsular invasion |
| Vascular invasion | Present in 10-15% cases tumor present within a vascular channel , partially attached to its wall | • Absent |
| Perineural invasion | • Rarely | • Absent |
| Infiltration | • thyroid, and adjacent tissue , distant metastases muscles, | • absent |
Table 2: Comparative table between histological features of parathyroid carcinoma and adenoma.
2) Atypical Adenoma
The term ‘‘atypical adenoma’’ has been used to describe a subset of parathyroid tumors that share some of the features of carcinomas (fibrosis, mitoses, questionable capsular invasion) but that lack unequivocal evidence of invasive growth [72]. According to Seethala et al [4], the presence of 2 or more of the following attributes will lead to this diagnosis: incomplete invasion of the capsule, fibrous bands, pronounced trabecular growth, mitotic activity greater than 1 per 10 high-power fields, and tumor necrosis [71].
There is apparently debate as to whether abnormal mitoses should be acceptable in the diagnosis of atypical adenoma; their presence should certainly lead to further investigation toward the elimination of malignancy [52]. Guiter and DeLellis [73] studied a series of 24 tumors that were classified as atypical adenomas based on the presence of peritumoral and intratumoral fibrosis, mitotic activity, questionable capsular invasion, and cytologic atypia. In this series, the mean tumor size was 2.2 cm with a mean weight of 6.5 g. The most common features in this group were entrapment of tumor within the capsule (87%), intratumoral fibrosis (75%), hemosiderin deposition (58%), cyst formation (50%), mitoses (29%), and peritumoral fibrosis (25%). Capsular invasion (without extension beyond the capsule) and intratumoral vascular invasion were each present in single cases, but none of the cases had evidence of necrosis. The average follow-up in this group was 4 years, and none of the patients has developed evidence of recurrent or metastatic disease. These findings suggest that the behavior of atypical adenomas, as defined in this study, does not differ from that of adenomas of usual type.
Treatment
Surgery
Surgery is the only curative treatment for parathyroid carcinoma and consists of complete resection of the primary lesion at the time of initial operation. For this reason, both preoperative suspicion and intraoperative recognition are of great importance [5]. The finding of a parathyroid tumor with gross features suggestive of malignancy should lead to “en bloc” resection. Generally, an open biopsy of such a lesion during surgery is contraindicated because of the risk of capsular rupture with seeding of the operative site [34].
As it often happens, the diagnosis of parathyroid carcinoma is made after initial parathyroid surgery on the basis of pathology, then, the management plan may become more problematic. When telling histologic features are absent, the patient is normocalcemic and the diagnosis is only based on equivocal pathology, immediate reoperation is not indicated, because the simple complete resection of the tumor may turn out to be curative. However, such patients should be monitored closely with regular measurement of serum calcium and PTH levels [48].
Chemotherapy
Several chemotherapy protocols have been attempted (vincristine, cyclophosphamide, and actinomycin D, and Adriamycin alone or in combination with cyclophosphamide and 5-fluorouracil), but none of them has proved to be effective [45,74]. Chemotherapy remains disappointing and has no role in the management of patients with parathyroid carcinoma.
Radiotherapy
In selected patients, adjuvant radiotherapy appears to decrease the rate of local recurrence [75] and may improve the diseasefree survival, particularly in high-risk patients. Radiofrequency ablation alone or in combination with arterial embolization has successfully been used in the treatment of hepatic and lung metastases in two patients with PC [76,77].
Prognosis
The prognosis of PC is variable. Patients with complete resection of the tumor at initial surgery carry the best prognosis. The mean time to recurrence is usually 3 years. Once the tumor recurs, a complete cure is unlike, although prolonged survival is still common with palliative surgery. A 5- and 10-year survival rate of 78.3 and 49%, respectively, has been reported [2]. Negative prognostic factors for survival were higher calcium level at recurrence, numbers of neck recurrences, the use of several calcium-lowering medications, and simple parathyroidectomy as initial surgery, presence of lymph nodes or distant metastases, and nonfunctioning PC. In addition, patients whose tumor carries a CDC73 mutation, and/or loss of parafibromin or CASR expression have a worse survival rate [2].
Conclusion
Parathyroid carcinoma is a rare disease, considered as the least common cause of primary hyperparathyroidism.
Because of the rarity of this tumor type, its biology, natural history, and prognosis are poorly understood
The disease is indolent but progressive. The most common presentation is complications of hypercalcaemia, but other ways of presentations are possible.
The main differential diagnosis is the parathyroid adenoma, and the differentiation between the two entities can be very difficult in some cases. Recent attempts to distinguish between benign and malignant disease both by genetic and immunohistochimical analyses are promising.
Given the lack of specific features, the diagnosis of parathyroid carcinoma continues to be a difficult challenge at the time of presentation. Thus, a multidisciplinary approach, considering all clinical, biochemical and histopathological aspects of the disease, offers the best chance for accurate diagnosis, before the appearance of metastases.
Invasion of the thyroid and or/adjacent soft tissues, and metastases remains the only unequivocal pathological features of parathyroid malignancy.
Surgery is the only curative option. The best opportunity to cure parathyroid carcinoma is to diagnose it before or at the time of parathyroid surgery and for the tumor to be completely removed at the time of the initial operation.
Other treatments such as chemotherapy, has not been reported to be effective, whereas radiotherapy may reduce the risk of recurrence.
References
- Marcocci C, Cetani F, Rubin MR, Silverberg SJ, Pinchera A, Bilezikian JP. Parathyroid carcinoma. Journal of Bone and Mineral Research. 2008 Dec;23(12):1869-80.
- Cetani F, Pardi E, Marcocci C. Update on parathyroid carcinoma. Journal of endocrinological investigation. 2016 Jun 1;39(6):595-606.
- De Quervain F. Parastruma maligna aberrata. Deutsche Zeitschrift Fuer Chirurgie. 1909 Jan 1;100(1):334-53.
- Sainton P. Millot J. Malegne dún adenoma parathyroidiene eosinophile; au cours dune de Recklinghausen. Annales Anatomie Pathologique. 1933;10:813.
- Shane E. Parathyroid carcinoma. The Journal of Clinical Endocrinology & Metabolism. 2001 Feb 1;86(2):485-93.
- Rawat N, Khetan N, Williams DW, Baxter JN. Parathyroid carcinoma. British journal of surgery. 2005 Nov 1;92(11):1345-53.
- Hundahl SA, Fleming ID, Fremgen AM, Menck HR. Two hundred eighty‐six cases of parathyroid carcinoma treated in the US between 1985–1995: a National Cancer Data Base Report. Cancer. 1999 Aug 1;86(3):538-44.
- Kebebew E. Parathyroid carcinoma. Current treatment options in oncology. 2001 Jul 1;2(4):347-54.
- Koea JB, Shaw JH. Parathyroid cancer: biology and management. Surgical oncology. 1999 Nov 1;8(3):155-65.
- Dajani AS, Taubert KA, Gerber MA, Shulman ST, Ferrieri P, Freed M, Takahashi M, Bierman FZ, Karchmer AW, Wilson W. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993 May;87(5):1776-80.
- Givi B, Shah JP. Parathyroid carcinoma. Clinical oncology. 2010 Aug 1;22(6):498-507.
- Kebebew E. Parathyroid carcinoma. Current treatment options in oncology. 2001 Jul 1;2(4):347-54.
- Boyle NH, Ogg CS, Hartley RB, Owen WJ. Parathyroid carcinoma secondary to prolonged hyperplasia in chronic renal failure and in coeliac disease. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 1999 Feb;25(1):100-3.
- Wassif WS, Moniz CF, Friedman EI, Wong S, Weber GU, Nordenskjöld M, Peters TJ, Larsson CA. Familial isolated hyperparathyroidism: a distinct genetic entity with an increased risk of parathyroid cancer. The Journal of Clinical Endocrinology & Metabolism. 1993 Dec 1;77(6):1485-9.
- Simonds WF, James-Newton LA, Agarwal SK, Yang B, Skarulis MC, Hendy GN, Marx SJ. Familial isolated hyperparathyroidism: clinical and genetic characteristics of 36 kindreds. Medicine. 2002 Jan 1;81(1):1-26.
- del Pozo C, García-Pascual L, Balsells M, Barahona MJ, Veloso E, González C, Anglada-Barceló J. Parathyroid carcinoma in multiple endocrine neoplasia type 1. Case report and review of the literature. Hormones. 2011 Oct 1;10(4):326-31.
- Jenkins PJ, Satta MA, Simmgen M, Drake WM, Williamson C, Lowe DG, Britton K, Chew SL, Thakker RV, Besser GM. Metastatic parathyroid carcinoma in the MEN2A syndrome. Clinical endocrinology. 1997 Dec;47(6):747-51.
- Kytölä S, Farnebo F, Obara T, Isola J, Grimelius L, Farnebo LO, Sandelin K, Larsson C. Patterns of chromosomal imbalances in parathyroid carcinomas. The American journal of pathology. 2000 Aug 1;157(2):579-86.
- Cryns VL, Thor A, Xu HJ, Hu SX, Wierman ME, Vickery Jr AL, Benedict WF, Arnold A. Loss of the retinoblastoma tumor-suppressor gene in parathyroid carcinoma. New England Journal of Medicine. 1994 Mar 17;330(11):757-61.
- Cetani F, Pardi E, Viacava P, Pollina GD, Fanelli G, Picone A, Borsari S, Gazzerro E, Miccoli P, Berti P, Pinchera A. A reappraisal of the Rb1 gene abnormalities in the diagnosis of parathyroid cancer. Clinical endocrinology. 2004 Jan;60(1):99-106.
- Cryns VL, Rubio MP, Thor AD, Louis DN, Arnold AN. p53 abnormalities in human parathyroid carcinoma. The Journal of Clinical Endocrinology & Metabolism. 1994 Jun 1;78(6):1320-4.
- Pearce SH, Trump D, Wooding C, Sheppard MN, Clayton RN, Thakker RV. Loss of heterozygosity studies at the retinoblastoma and breast cancer susceptibility (BRCA2) loci in pituitary, parathyroid, pancreatic and carcinoid tumours. Clinical endocrinology. 1996 Aug;45(2):195-200.
- Shattuck TM, Kim TS, Costa J, Yandell DW, Imanishi Y, Palanisamy N, Gaz RD, Shoback D, Clark OH, Monchik JM, Wierman ME. Mutational analyses of RB and BRCA2 as candidate tumour suppressor genes in parathyroid carcinoma. Clinical endocrinology. 2003 Aug;59(2):180-9.
- Arnold A, Shattuck TM, Mallya SM, Krebs LJ, Costa J, Gallagher J, Wild Y, Saucier K. Molecular pathogenesis of primary hyperparathyroidism. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2002 Nov;17:N30-6.
- Arnold A, Shattuck TM, Mallya SM, Krebs LJ, Costa J, Gallagher J, Wild Y, Saucier K. Molecular pathogenesis of primary hyperparathyroidism. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2002 Nov;17:N30-6.
- Haven CJ, Van Puijenbroek M, Tan MH, Teh BT, Fleuren GJ, Van Wezel T, Morreau H. Identification of MEN1 and HRPT2 somatic mutations in paraffin‐embedded (sporadic) parathyroid carcinomas. Clinical endocrinology. 2007 Sep;67(3):370-6.
- Cavaco BM, Barros L, Pannett AA, Ruas L, Carvalheiro M, Ruas MM, Krausz T, Santos MA, Sobrinho LG, Leite V, Thakker RV. The hyperparathyroidism‐jaw tumour syndrome in a Portuguese kindred. Qjm. 2001 Apr 1;94(4):213-22.
- Cetani F, Ambrogini E, Viacava P, Pardi E, Fanelli G, Naccarato AG, Borsari S, Lemmi M, Berti P, Miccoli P, Pinchera A. Should parafibromin staining replace HRTP2 gene analysis as an additional tool for histologic diagnosis of parathyroid carcinoma?. European Journal of Endocrinology. 2007 May 1;156(5):547-54.
- Zhang C, Kong D, Tan MH, Pappas Jr DL, Wang PF, Chen J, Farber L, Zhang N, Koo HM, Weinreich M, Williams BO. Parafibromin inhibits cancer cell growth and causes G1 phase arrest. Biochemical and biophysical research communications. 2006 Nov 10;350(1):17-24.
- Mosimann C, Hausmann G, Basler K. Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with β-catenin/Armadillo. Cell. 2006 Apr 21;125(2):327-41.
- Hewitt KM, Sharma PK, Samowitz W, Hobbs M. Aberrant methylation of the HRPT2 gene in parathyroid carcinoma. Annals of Otology, Rhinology & Laryngology. 2007 Dec;116(12):928-33.
- Krebs LJ, Shattuck TM, Arnold A. HRPT2 mutational analysis of typical sporadic parathyroid adenomas. The Journal of Clinical Endocrinology & Metabolism. 2005 Sep 1;90(9):5015-7.
- Senior K. Vital gene linked to parathyroid carcinoma. The Lancet Oncology. 2003 Dec 1;4(12):713.
- Dudney WC, Bodenner D, Stack BC. Parathyroid carcinoma. Otolaryngologic clinics of North America. 2010 Apr 1;43(2):441-53.
- Fernandez-Ranvier G, Jensen K, Khanafshar E, Quivey J, Glastonbury C, Kebebew E, Duh QY, Clark O. Nonfunctioning parathyroid carcinoma: case report and review of literature. Endocrine Practice. 2007 Nov 1;13(7):750-7.
- Talat N, Schulte KM. Clinical presentation, staging and long-term evolution of parathyroid cancer. Annals of surgical oncology. 2010 Aug 1;17(8):2156-74.
- Whitson BA, Broadie TA. Preoperative ultrasound and nuclear medicine studies improve the accuracy in localization of adenoma in hyperparathyroidism. Surgery Today. 2008 Mar 1;38(3):222-6.
- Vickers NJ. Animal communication: when i’m calling you, will you answer too?. Current biology. 2017 Jul 24;27(14):R713-5.
- Duan K, Mete Ö. Parathyroid carcinoma: diagnosis and clinical implications. Turkish Journal of Pathology. 2015 May 2;31.
- Obara T, Fujimoto Y. Diagnosis and treatment of patients with parathyroid carcinoma: an update and review. World journal of surgery. 1991 Nov 1;15(6):738-44.
- Rubello D, Casara D, Dwamena BA, Shapiro B. Parathyroid carcinoma. A concise review. Minerva endocrinologica. 2001 Jun;26(2):59.
- Brown JJ, Mohamed H, Williams-Smith L, Osborne R, Coker J, Yee B. Primary hyperparathyroidism secondary to simultaneous bilateral parathyroid carcinoma. Ear, nose & throat journal. 2002 Jun;81(6):395-401.
- Cohn K, Silverman M, Corrado J, Sedgewick C. Parathyroid carcinoma: the Lahey Clinic experience. Surgery. 1985 Dec 1;98(6):1095-100.
- Flye MW, Brennan MF. Surgical resection of metastatic parathyroid carcinoma. Annals of surgery. 1981 Apr;193(4):425.
- Wynne AG, Van Heerden J, Carney JA, Fitzpatrick LA. Parathyroid carcinoma: clinical and pathologic features in 43 patients. Medicine. 1992 Jul 1;71(4):197-205.
- Rawat N, Khetan N, Williams DW, Baxter JN. Parathyroid carcinoma. British journal of surgery. 2005 Nov 1;92(11):1345-53.
- Dionisi S, Minisola S, Pepe J, De Geronimo S, Paglia F, Memeo L, Fitzpatrick LA. Concurrent parathyroid adenomas and carcinoma in the setting of multiple endocrine neoplasia type 1: presentation as hypercalcemic crisis. InMayo Clinic Proceedings 2002 Aug 1 (Vol. 77, No. 8, pp. 866-869). Elsevier.
- Marcocci C, Cetani F. rubin Mr, Silverberg SJ, Pinchera a, Bilezikian JP, 2008 Parathyroid carcinoma. J Bone Min res.;23:1868-80.
- DeLellis RA. Challenging lesions in the differential diagnosis of endocrine tumors: parathryoid carcinoma. Endocrine pathology. 2008 Dec 1;19(4):221.
- Nacamuli R, Rumore GJ, Clark G. Parathyroid carcinosarcoma: a previously unreported entity. The American surgeon. 2002 Oct 1;68(10):900.
- Tan MH, Morrison C, Wang P, Yang X, Haven CJ, Zhang C, Zhao P, Tretiakova MS, Korpi-Hyovalti E, Burgess JR, Soo KC. Loss of parafibromin immunoreactivity is a distinguishing feature of parathyroid carcinoma. Clinical Cancer Research. 2004 Oct 1;10(19):6629-37.
- Stojadinovic A, Hoos A, Nissan A, Dudas ME, Cordon-Cardo C, Shaha AR, Brennan MF, Singh B, Ghossein RA. Parathyroid neoplasms: clinical, histopathological, and tissue microarray-based molecular analysis. Human pathology. 2003 Jan 1;34(1):54-64.
- Wang CA, Gaz RD. Natural history of parathyroid carcinoma: diagnosis, treatment, and results. The American journal of surgery. 1985 Apr 1;149(4):522-7.
- Schantz A, Castleman B. Parathyroid carcinoma. A study of 70 cases. Cancer. 1973 Mar;31(3):600-5.
- Bondeson L, Sandelin K, Grimelius L. Histopathological variables and DNA cytometry in parathyroid carcinoma. The American journal of surgical pathology. 1993 Aug;17(8):820-9.
- McKeown PP, McGarity WC, Sewell CW. Carcinoma of the parathyroid gland: is it overdiagnosed?: A report of three cases. The American journal of surgery. 1984 Feb 1;147(2):292-8.
- Sheffield EA. An approach to common pathological conditions in the parathyroid glands. CPD Cell Pathol. 2002;4:135-8.
- Snover DC, Foucar K. Mitotic activity in benign parathyroid disease. American Journal of Clinical Pathology. 1981 Mar 1;75(3):345-7.
- San Juan J, Monteagudo C, Fraker D. Significance of mitotic activity and other morphological parameters in parathyroid adenomas and their correlation with clinical behavior. Am J Clin Pathol. 1989;92:523.
- Johnson SJ. Changing clinicopathological practice in parathyroid disease. Histopathology. 2010 Jun;56(7):835-51.
- Campennì A, Ruggeri RM, Sindoni A, Giovinazzo S, Calbo E, Ieni A, Calbo L, Tuccari G, Baldari S, Benvenga S. Parathyroid carcinoma presenting as normocalcemic hyperparathyroidism. Journal of bone and mineral metabolism. 2012 May 1;30(3):367-72.
- Erickson LA, Lloyd RV. Practical markers used in the diagnosis of endocrine tumors. Advances in Anatomic Pathology. 2004 Jul 1;11(4):175-89.
- DeLellis RA. Challenging lesions in the differential diagnosis of endocrine tumors: parathryoid carcinoma. Endocrine pathology. 2008 Dec 1;19(4):221.
- Rubin MR, Silverberg SJ. Editorial: HRPT2 in parathyroid cancer: a piece of the puzzle. J Clin Endocrinol Metab. 2005 Sep;90(9):5505-7.
- Tan MH, Morrison C, Wang P, Yang X, Haven CJ, Zhang C, Zhao P, Tretiakova MS, Korpi-Hyovalti E, Burgess JR, Soo KC. Loss of parafibromin immunoreactivity is a distinguishing feature of parathyroid carcinoma. Clinical Cancer Research. 2004 Oct 1;10(19):6629-37.
- Gill AJ, Clarkson A, Gimm O, Keil J, Dralle H, Howell VM, Marsh DJ. Loss of nuclear expression of parafibromin distinguishes parathyroid carcinomas and hyperparathyroidism-jaw tumor (HPT-JT) syndrome-related adenomas from sporadic parathyroid adenomas and hyperplasias. The American journal of surgical pathology. 2006 Sep 1;30(9):1140-9.
- Juhlin CC, Villablanca A, Sandelin K, Haglund F, Nordenstrom J, Forsberg L, Branstrom R, Obara T, Arnold A, Larsson C, Hoog A. Parafibromin immunoreactivity: its use as an additional diagnostic marker for parathyroid tumor classification. Endocrine-related cancer. 2007 Jun 1;14(2):501-12.
- Juhlin C, Larsson C, Yakoleva T, Leibiger I, Leibiger B, Alimov A, Weber G, Hoog A, Villablanca A. Loss of parafibromin expression in a subset of parathyroid adenomas. Endocrine-Related Cancer. 2006 Jun 1;13(2):509-23.
- Ryhänen EM, Leijon H, Metso S, Eloranta E, Korsoff P, Ahtiainen P, Kekäläinen P, Tamminen M, Ristamäki R, Knutar O, Löyttyniemi E. A nationwide study on parathyroid carcinoma. Acta Oncologica. 2017 Jul 3;56(7):991-1003.
- Lloyd RV, Carney JA, Ferreiro JA, Jin L, Thompson GB, van Heerden JA, Grant CS, Wollan PC. Immunohistochemical analysis of the cell cycle-associated antigens Ki-67 and retinoblastoma protein in parathyroid carcinomas and adenomas. Endocrine Pathology. 1995 Nov 1;6(4):279-87.
- Carlson D. Parathyroid pathology: hyperparathyroidism and parathyroid tumors. Archives of Pathology and Laboratory Medicine. 2010 Nov;134(11):1639-44.
- Levin KE, CHEW KL, LJUNG BM, MAYALL BH, SIPERSTEIN AE, CLARK OH. Deoxyribonucleic acid cytometry helps identify parathyroid carcinomas. The Journal of Clinical Endocrinology & Metabolism. 1988 Oct 1;67(4):779-84.
- Guiter GE, DeLellis RA. Risk of recurrence or Metastases in atypical parathyroid adenomas (APTAs). InLABORATORY INVESTIGATION 2002 Jan 1 (Vol. 82, No. 1, pp. 115A-115A). 75 VARICK ST, 9TH FLR, NEW YORK, NY 10013-1917 USA: NATURE PUBLISHING GROUP.
- Rao SR, Shaha AR, Singh B, Rinaldo A, Ferlito A. Management of cancer of the parathyroid. Acta oto-laryngologica. 2002 Jan 1;122(4):448-52.
- Clayman GL, Gonzalez HE, El‐Naggar A, Vassilopoulou‐Sellin R. Parathyroid carcinoma: evaluation and interdisciplinary management. Cancer. 2004 Mar 1;100(5):900-5.
- Steer CB, Marx GM, Galani E, Harper PG, Khayat D. Quality of life: It’s never too late.
- Tochio M, Takaki H, Yamakado K, Uraki J, Kashima M, Nakatsuka A, Takao M, Shimamoto A, Tarukawa T, Shimpo H, Takeda K. A case report of 20 lung radiofrequency ablation sessions for 50 lung metastases from parathyroid carcinoma causing hyperparathyroidism. Cardiovascular and interventional radiology. 2010 Jun 1;33(3):657-9.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences