Histone Deacetylases Inhibition on Diabetes Mellitus Control: A promising, but long way to go
Ting-I Lee, Yu-Hsun Kao and Yi-Jen Chen
Ting-I Lee1,2, Yu-Hsun Kao3,4 and Yi-Jen Chen3,5*
1Department of General Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
2Division of Endocrinology and Metabolism, Department of Internal Medicine, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
3Graduate Institute of Clinical Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
4Department of Medical Education and Research, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
5Division of Cardiovascular Medicine, Department of Internal Medicine, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan
- *Corresponding Author:
- Chen YJ
Graduate Institute of Clinical Medicine
College of Medicine
Taipei Medical University, Taiwan
Tel: +886228757156
Fax: +886229339378
E-mail: a9900112@ms15.hinet.net
Received date: September 19, 2016; Accepted date: October 04, 2016; Published date: October 07, 2016
Citation: Lee TI, Kao YH, Chen YJ (2016) Histone Deacetylases Inhibition on Diabetes Mellitus Control: A promising, but Long Way to go. J Clin Mol Endocrinol 1:27. doi: 10.21767/2572-5432.100025
Copyright: © 2016 Lee TI, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abbreviations
IRS-1: Insulin receptor substrate-1; GLUT: Glucose transporter type 4; PDX: Pancreatic and duodenal homeobox 1; FOXO: Forkhead box protein O1; IL-1β: Interleukin-1β; IFN-γ: Interferon gamma; TFN-α: Tumor necrosis factor-α; TGFβ: Transforming growth factor beta 1; ER: Endoplasmic reticulum; eNOS: Endothelial nitric oxide synthase; Pgc-1α: Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; Pgc-1β: Peroxisome proliferator-activated receptor gamma coactivator 1-beta; Tfam: Mitochondrial transcription factor A; Tfb1m: Transcription factor B1 mitochondrial; Cytc: Cytochrome complex; Cox6a: Cytochrome c oxidase 6a1; Etfdh: Electron transferring flavoprotein dehydrogenase; NEFA: Non-esterified fatty acid; FGF: Fibroblast growth factor 21; PPARs: Peroxisome proliferator-activated receptors.
Letter to Editor
Diabetes mellitus (DM) is a chronic disease affecting 285 million individuals worldwide, and is estimated to increase by the year 2030 to 439 million patients [1]. The effective treatment in reducing DM risk is insufficient since the pathophysiology of DM cardiomyopathy is not well established. Emerging concepts have link epigenetic mechanisms in the regulation of DM and its cardiovascular complications, which are the leading causes of DM mortality or morbidities. Among them, histone deacetylases (HDACs) play a crucial role in the transcription of genes to modulate proliferation, migration and death of cells [2]. HDACs are enzymes that compete with histone acetyltransferase to regulate the acetylation of lysine residues during chromatin remodeling [3]. Recent studies indicate that targeting HDACs is a promising therapeutic strategy for a number of other diseases such as DM, as demonstrated in cellular and animal models [4-7], and DM complications [8-11].
We recently evaluated the effect of HDAC inhibitor, MPT0E01, in a streptozotocin-nicotinamide induced DM rat cardiomyocytes, and found that this pan-HDAC inhibitor significantly attenuated DM cardiomyopathy through modulation of cardiac peroxisome proliferator-activated receptors (PPARs), fatty acid metabolism, and proinflammatory cytokines [12]. Similarly, MPT0E014 was shown to suppress cardiac fibrosis [13], and modulate cardiac PPARs, and inflammatory cytokines in a heart failure [14]. The effect of MPT0E014 on cardiac PPARs may have resulted from its antiinflammatory activity. Besides, MPT0E014 may modulate cardiac metabolism through its effects on PPARs and inflammatory cytokines to diminish the accumulation of fatty acids in DM hearts.
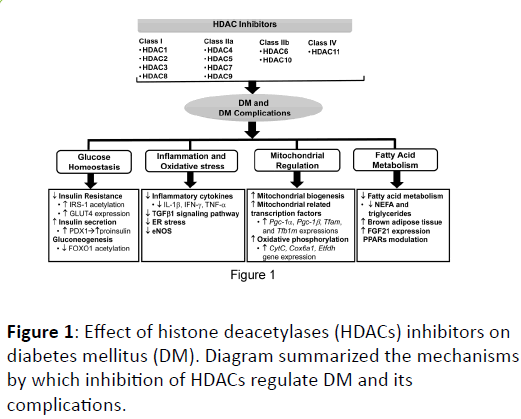
Although laboratory evidences support the potential role of HDAC inhibition on DM and its complications, the precise mechanisms underlying the effects of HDAC inhibitors are not clear. Several possibilities at least are expected to involve the effects of HDAC inhibition on DM control, which includes glucose homeostasis, inflammation and oxidative stress, fatty acid metabolisms, and mitochondria regulation (Figure 1).
In addition, the individualized classes of HDAC inhibition responsible for their distinctive role in DM control are not elucidated. It is not clear whether MPT0E014 has direct effects on DM cardiac metabolism by directly measuring carbohydrate and fatty acid utilization. In addition, the downstream signaling pathways underlying the activity of MPT0E014 on myocardial inflammatory cytokines and PPAR expressions, the direct impact of MPT0E014 on PPAR gene expressions, and the effects of MPT0E014 on the regulation of myocardial autophagy need further investigations. It is mandatory to study the effects of HDAC inhibition in more clinically relevant type 2 DM model (not from streptozotocin-induction) and evaluate their effects in other organs related to glucose homeostasis such as pancreas, liver, muscles and adipose tissues.
In conclusion, the HDAC inhibition is a promising strategy in controlling DM and its complications. But it still has a long way to go due to several curious questions remaining unsolved.
References
- Shaw JE,Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res ClinPract 87: 4-14.
- Christensen DP, Dahllof M, Lundh M, Rasmussen DN, Nielsen MD, et al. (2011) Histone deacetylase (HDAC) inhibition as a novel treatment for diabetes mellitus. Molecular medicine 17:378-390.
- Verdin E,Dequiedt F, Kasler HG (2003) Class II histone deacetylases: versatile regulators. Trends Genet 19: 286-293.
- Larsen L,TonnesenM, Ronn SG, Storling J, Jorgensen S, et al. (2007) Inhibition of histone deacetylases prevents cytokine-induced toxicity in beta cells. Diabetologia 50: 779-789.
- Lundh M, Christensen DP, Rasmussen DN, Mascagni P, Dinarello CA, et al. (2010) Lysine deacetylases are produced in pancreatic beta cells and are differentially regulated by proinflammatory cytokines. Diabetologia 53:2569-2578.
- Susick L, Senanayake T, Veluthakal R, Woster PM, Kowluru A (2009) A novel histone deacetylase inhibitor prevents IL-1beta induced metabolic dysfunction in pancreatic beta-cells. J Cell Mol Med 13:1877-1885.
- Galmozzi A, Mitro N, Ferrari A, Gers E, Gilardi F, et al. (2013) Inhibition of class I histone deacetylases unveils a mitochondrial signature and enhances oxidative metabolism in skeletal muscle and adipose tissue. Diabetes 62:732-742.
- Advani A, Huang Q, Thai K, Advani SL, White KE, et al. (2011) Long-term administration of the histone deacetylase inhibitor vorinostat attenuates renal injury in experimental diabetes through an endothelial nitric oxide synthase-dependent mechanism. Am J Pathol178:2205-2214.
- Noh H, Oh EY, Seo JY, Yu MR, Kim YO, et al. (2009) Histone deacetylase-2 is a key regulator of diabetes- and transforming growth factor-beta1-induced renal injury. Am J Physiol Renal Physiol297:F729-739.
- Crosson CE, Mani SK, Husain S, Alsarraf O, Menick DR (2010) Inhibition of histone deacetylase protects the retina from ischemic injury. Invest Ophthalmol VisSci 51:3639-3645.
- Zhong Q,Kowluru RA (2010) Role of histone acetylation in the development of diabetic retinopathy and the metabolic memory phenomenon. J Cell Biochem 110: 1306-1313.
- Lee TI, Kao YH, Tsai WC, Chung CC, Chen YC, et al. (2016) HDAC inhibition modulates cardiac PPARs and fatty acid metabolism in diabetic cardiomyopathy. PPAR Res 2016: 5938740.
- Kao YH, Liou JP, Chung CC, Lien GS, Kuo CC, et al. (2013) Histone deacetylase inhibition improved cardiac functions with direct antifibrotic activity in heart failure. Int J Cardiol168:4178-4183.
- Lkhagva B, Lin YK, Kao YH, Chazo TF, Chung CC, et al. (2015) Novel histone deacetylase inhibitor modulates cardiac peroxisome proliferator-activated receptors and inflammatory cytokines in heart failure. Pharmacology 96:184-191.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences