Effect of Genistein on Endometriosis Lesion, Matrix Metalloproteinase-2 and -9 Level of Endometriosis: In silico and In vivo Study
Maharani Maharani, Endang Sri Wahyuni , Sutrisno Sutrisno
Maharani Maharani1,2, Endang Sri Wahyuni3and Sutrisno Sutrisno4*
1Midwifery Master Study Programme, Faculty of Medicine, Brawijaya University, Malang, East Java, Indonesia
2Health Polytechnical, Ministry of Health, Aceh, Indonesia
3Physiology Molecular Laboratory, Faculty of Medicine, Brawijaya University, Malang, East Java, Indonesia
4Division of Fertility, Endocrinology and Reproduction, Department of Obstetric and Ginaecology, Saiful Anwar General Hospital, Faculty of Medicine, Brawijaya University, Malang, East Java, Indonesia
- *Corresponding Author:
- Sutrisno S
Division of Fertility, Endocrinology and Reproduction
Department of Obstetric and Ginaecology, Saiful Anwar General Hospital
Faculty of Medicine, Brawijaya University, Malang, East Java, Indonesia
E-mail: snospogk@gmail.com
Received date: December 28, 2015 Accepted date: January 11, 2016 Published date: January 19, 2016
Citation: Sutrisno S, Maharani M, Sri Wahyuni E (2016) Effect of Genistein on Endometriosis Lesion, Matrix Metalloproteinase-2 and -9 Level of Endometriosis: In silico and In vivo Study. J Clin Mol Endocrinol 1:4. doi: 10.21767/2572-5432.100004
Copyright: © 2016 Maharani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
This in silico and in vivo investigation were to know the effect of genistein on alteration of MMPs in a mice model of endometriosis. Forty female mice (Mus musculus) were divided into eight groups (n=5 each), including the control (untreated) group, endometriosis group, and the endometriosis (EM) groups were given various doses of genistein (at dose of 50; 100; 200; 300; 400; 500 mg/day). In silico analysis was performed using Open Babel, 3D DART webserver, HEX software, and NUCPLOT software. Analysis of MMP-2 and MMP-9 level were done by enzyme linked immuno sorbent assay (ELISA) technically. In the absence of genistein, ΣE total required for the interaction between NF-?B with MMP-2 or MMP-9 gene promoter were increased in genistein treatment (-2393.71 kJ/mol and -2393.86 kJ/mol) compared with no genistein treatment (-2415.62 kJ/mol and -2457.00 kJ/mol). In endometriosis group we were founded the massive endometriosis lesion and hyper vascularization. All doses the treatment with genistein reduces the size of endometriosis lesion. The level of MMP-2 and MMP-9 was significantly higher in the EM group compared to the untreated control group (P<0.05). This increase in MMP-2 was significantly (P<0.05) attenuated by all doses of genistein. These increased levels of MMP-9 in the EM group were significantly reduced by 100 mg/day administration of genistein. In conclusion, genistein prohibits the increase in MMP-2 and MMP-9 in a mice model of endometriosis. Therefore, this may provide a natural therapy for attenuating the alteration of MMPs found in endometriosis disease.
Keywords
Isoflavone; Peritoneum; Matrix metalloproteinase; Enzyme; Bioinformatic
Introduction
Endometriosis is a benign gynecological disorder characterized by the growth of endometrial glands and stroma outside the uterine cavity (grown in ectopic). Endometriosis is a disability for women of reproductive age and is very rare in adolescent or post-menopause [1-4]. This disorder is the cause of the symptoms of pain (dysmenorrhea, dyspareunia, chronic pelvic pain) and infertility [1-5].
The prevalence of endometriosis in the population is difficult to determine due to asymptomatic or subclinical cases. In women with symptoms of pain, the prevalence of endometriosis is higher, around 10-60%. For overall prevalence, for 4-19% of all women and only 1% in asymptomatic women [3,4,6,7].
The Sampson's theory proposes that the disorder caused by a retrograde menstruation of viable endometrial tissue through the Fallopian tube into the peritoneal cavity and then implant to the peritoneal surface of the pelvic organs [8]. Various models of non-primates and primates have been developed for the study of endometriosis.
Monkey models cannot be denied as the most relevant model of physiologically due to the emergence of spontaneous endometriosis [9]. Given the practical and ethical aspects of research, several researchers have also developed a model of endometriosis in small mammals, such as rabbits, rats, and mice [10-15].
The process of tumor cell invasion involves various stages mediated by tumor-derived genes and gene derivatives host. Tumor cells are characterized by uncontrolled growth, high migration capacity, and capability for the production of tumor protease to support the proliferation of tumors [16,17]. One protease associated with the invasive capacity of tumor cells is the matrix metalloproteinases (MMPs) [18].
Previous research proved that polymorphisms of MMP-2 (-735 C/T) and MMP-9 (-1562 C/T) is associated with an increased risk of endometriosis [19]. In addition, it has been revealed that the increase in MMP-2 and MMP-9 plays a role in the pathogenesis of endometriosis, especially in the behavior of the invasion and metastasis [20].
Genistein is a people’s daily diet as antitumor function [21,22]. There is a correlation between high levels of genistein urine with decreased risk of endometriosis. Genistein (at 500 mg/kg day orally for 21 days) also trigger endometriosis implantation regression, which marked by decreasing size of lesion in rat model of endometriosis [23,24].
Previous studies in breast cancer have shown that genistein, both in vivo and in vitro, down-regulates the expression of MMPs by inhibiting HER-2/neu receptor phosphorylation and PTK activity [25]. Despite the possible benefits of genistein have increasingly attracted more attention, little is known regarding the effect of genistein on MMPs expression in mice endometriosis model. Therefore, this in silico and in vivo study was undertaken to investigate the effect of genistein on alteration MMPs expression in a mice model of endometriosis.
Materials and Methods
Animal
Forty female mice (Mus musculus), aged 2-3 months, weighing 20-30 grams were divided into eight groups, including the control (untreated) group, endometriosis group, and the endometriosis groups were given various doses of genistein (at doses of 50; 100; 200; 300; 400; 500 mg/day).
Mus musculus was obtained from the breeding facility at the Laboratory of Reproductive Physiology, Embryology, Faculty of Veterinary Medicine, Airlangga University, Surabaya. This study was conducted at Physiology Laboratory, Faculty of Medicine, University of Brawijaya, Malang.
Genistein treatment
Genistein (Tokyo Chemical Industries, Japan) powder was dissolved in sesame oil (1 ml volume containing 1 gram). Genistein treatment was performed after 14 days of induction in mice model of endometriosis [24,26]. Genistein solution was administered orally for 14 days with an oral gavage.
Endometriosis model
In immunodeficient mice, the implantation of the myometrium and endometrium tissue implantation will produce a mice model of endometriosis. Cyclosporin A (0.2 ml/ mice, single dosage) injection was given intraperitoneally to make mice in immunodeficient state. Implant tissue derived from the myometrium and endometrium of adenomysosis patient (1 patient) who underwent surgery procedure.
The patient already signed the approval for research. The uterus tissue (1 cm3) of benign tumors of the uterus (adenomyosis) was washed twice at 3000 RPM at 4°C. Supernatant was discarded, and then added to PBS.
Implantation is done by injection (0.1 ml) in the peritoneal cavity. The intramuscular ethynil estradiol injections (0.1 ml/ mice) were performed on day one and five. The mice model was observed for 14 days to be the mice model of endometriosis [27].
Peritoneal fluid collection
At the end of the experiment, mice were anesthetized with diethyl ether, and then peritoneal fluid samples were obtained by peritoneum puncture. The fluid was separated by 5 min of centrifugation at 3000 RPM and 4°C. All samples were stored at −80°C until analyzed.
Determination of MMP-2
The Mouse MMP-2 ELISA kit was purchased from Elabscience Biotechnology Co., Ltd (Wuhan, Hubei Province, PR of China). The analysis was done according to detail procedures in the kit.
Determination of MMP-9
The Mouse MMP-2 ELISA kit was purchased from Elabscience Biotechnology Co., Ltd (Wuhan, Hubei Province, PR of China). The analysis was done according to detail procedures in the kit.
In Silico Analysis
MMP2 gene promoter sequences (GI: 38,194,199) and MMP9 (GI: 15,420,889) were obtained from the database of the National Center for Biotechnology Information (NCBI), the United States National Library of Medicine (NLM), National Institutes of Health (NIH) [www.ncbi.nlm.nih.gov]. 3D structure of the transcription factor NFκB was predicted by SWISS-MODEL webserver via homology modeling [28].
3D structure of the compound was obtained from PubChem Compound genistein (PubChem CID: 5280961), and the conversion of the * .sdf file into * .pdb files were done by using the software Open Babel. The 3D structure of MMP2 and MMP9 gene promoters were predicted by 3D DART webserver [29].
Furthermore, the process of docking between NFkB with MMP2 and MMP9 gene promoter was done with HEX software and the interaction analysis was performed using NUCPLOT software [30,31].
Statistical Analysis
Data are presented as mean ± SD and the differences between groups were analyzed using one-way analysis of variance (ANOVA) with SPSS 15.0 statistical package for Windows. Only probability values of P<0.05 were considered statistically significant and later subjected to Tukey’s post hoc test.
Results
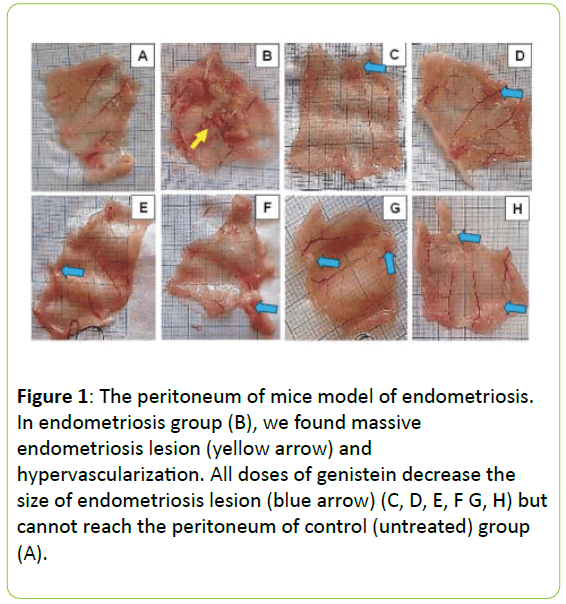
In endometriosis group we were founded the massive endometriosis lesion and hypervascularization. All doses the treatment with genistein reduces the size of endometriosis lesion, as seen in Figure 1.
Figure 1: The peritoneum of mice model of endometriosis. In endometriosis group (B), we found massive endometriosis lesion (yellow arrow) and hypervascularization. All doses of genistein decrease the size of endometriosis lesion (blue arrow) (C, D, E, F G, H) but cannot reach the peritoneum of control (untreated) group (A).
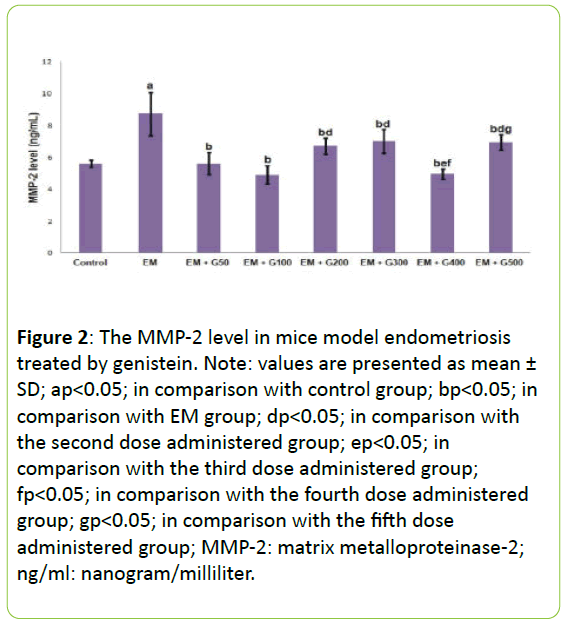
Figure 2 presents the MMP-2 levels in the peritoneal fluid from each experimental group. The level of MMP-2 was significantly higher in the EM group compared to the untreated control group (P<0.05). All doses genistein significantly prevented EM-induced increase in MMP-2 level, to reach the level in the control group.
Figure 2: The MMP-2 level in mice model endometriosis treated by genistein. Note: values are presented as mean ± SD; ap<0.05; in comparison with control group; bp<0.05; in comparison with EM group; dp<0.05; in comparison with the second dose administered group; ep<0.05; in comparison with the third dose administered group; fp<0.05; in comparison with the fourth dose administered group; gp<0.05; in comparison with the fifth dose administered group; MMP-2: matrix metalloproteinase-2; ng/ml: nanogram/milliliter.
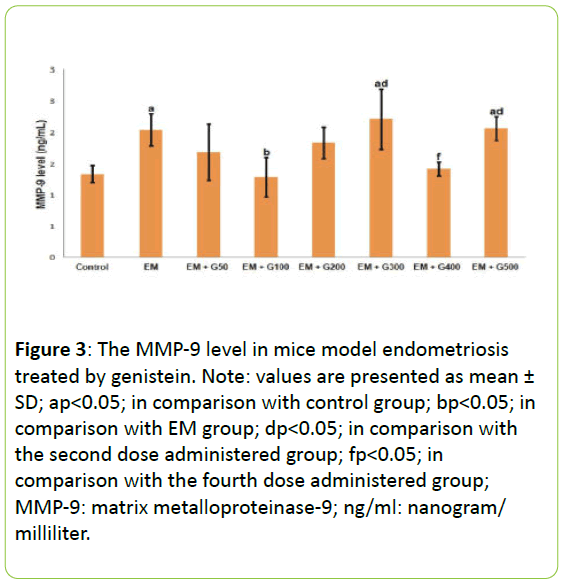
Figure 3 presents the MMP-9 levels in the peritoneal fluid from each experimental group. The MMP-9 levels were significantly greater in the EM group compared to the untreated group. These increased levels of MMP-9 in the EM group were significantly reduced by 100 mg/day administration of genistein than that another dose. Indeed, administration of 100 mg/day to the EM group reduced MMP-2 levels to those comparable to the untreated group.
Figure 3: The MMP-9 level in mice model endometriosis treated by genistein. Note: values are presented as mean ± SD; ap<0.05; in comparison with control group; bp<0.05; in comparison with EM group; dp<0.05; in comparison with the second dose administered group; fp<0.05; in comparison with the fourth dose administered group; MMP-9: matrix metalloproteinase-9; ng/ml: nanogram/ milliliter.
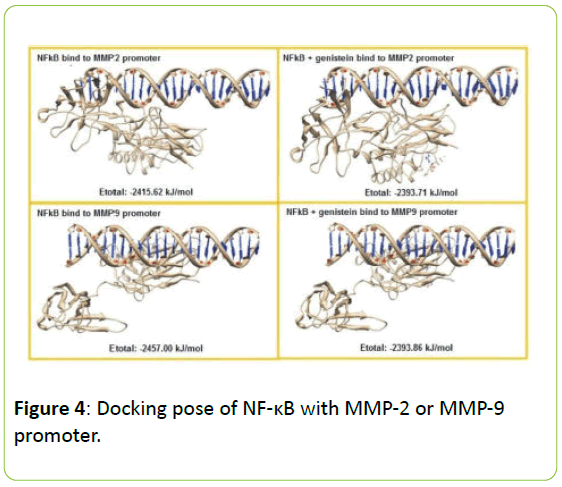
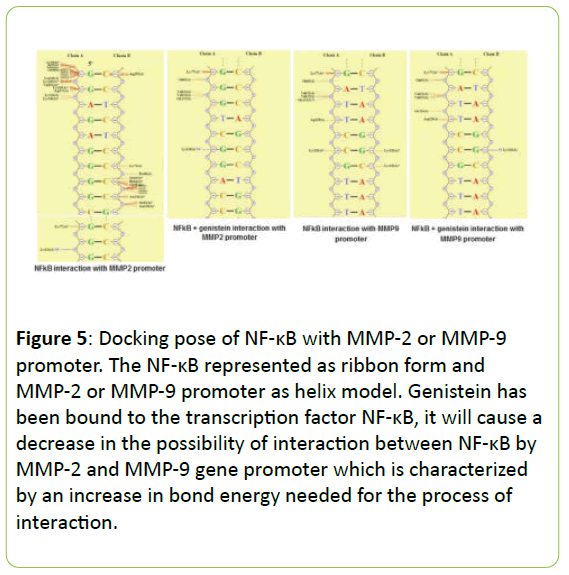
Figure 4 present the interaction between NF-κB the MMP-2 and MMP-9 gene promoter with or without genistein treatment. In the absence of genistein, this interaction was form a hydrogen bond between G4622 (A) → Lys114, two van der Waals interactions between the C4671 (B) → Arg252; C4664 (B) → Ser63 and a lot of contact without bond formed between G4611 (A) → Leu 248, Lys249, Ile250, Val251, Leu269, Cys270; G4612 (A) → Lys241, Lys249, Asp271; G4621 (A) → Lys77; C4665 (B) → Lys74, Pro68; C4664 (B) → Ser63, His64, Gly65; C4663 (B) → Asn136, Ser63, Asn136.
When the genistein presences, the interaction process is also formed a hydrogen bond between G4626 (A) → Lys128 and a lot of contact without bond formed between G4619 (A) → Asn75; C4620 (A) → Lys76, Lys77; G4621 (A) → Gln81; G4622 (A) → Val131, Val132, Gly133; C4666 (B) → Lys74; C4665 (B) → Lys77; C4664 (B) → Gly66, Asn136; C4663 (B) → Lys114, Asn136, but does not form van der Waals interactions at all. In addition, the interaction between NF-κB the MMP-9 gene promoter when no genistein, occurred one hydrogen bond between A1282 (A) → Gly133, and occurs four van der Waals interactions between the T1284 (A) → Asp129; C1286 (A) → Lys128; G1316 (B) → Lys128; and C1315 (B) → Lys128. When there are genistein, only one hydrogen bond is formed between G1316 (B) → Lys128 and the van der Waals bonding between T1284 (A) → Asp129.
In the absence of genistein, this interaction was form a hydrogen bond between G4622 (A) → Lys114, two van der Waals interactions between the C4671 (B) → Arg252; C4664 (B) → Ser63 and a lot of contact without bond formed between G4611 (A) → Leu 248, Lys249, Ile250, Val251, Leu269, Cys270; G4612 (A) → Lys241, Lys249, Asp271; G4621 (A) → Lys77; C4665 (B) → Lys74, Pro68; C4664 (B) → Ser63, His64, Gly65; C4663 (B) → Asn136, Ser63, Asn136.
When the genistein presences, the interaction process is also formed a hydrogen bond between G4626 (A) → Lys128 and a lot of contact without bond formed between G4619 (A) → Asn75; C4620 (A) → Lys76, Lys77; G4621 (A) → Gln81; G4622 (A) → Val131, Val132, Gly133; C4666 (B) → Lys74; C4665 (B) → Lys77; C4664 (B) → Gly66, Asn136; C4663 (B) → Lys114, Asn136, but does not form van der Waals interactions at all. In addition, the interaction between NF-κB the MMP-9 gene promoter when no genistein, occurred one hydrogen bond between A1282 (A) → Gly133, and occurs four van der Waals interactions between the T1284 (A) → Asp129; C1286 (A) → Lys128; G1316 (B) → Lys128; and C1315 (B) → Lys128. When there are genistein, only one hydrogen bond is formed between G1316 (B) →s Lys128 and the van der Waals bonding between T1284 (A) → Asp129.
In the absence of genistein, ΣE total required for the interaction between NF-κB with MMP-2 or MMP-9 gene promoter was -2415.62 kJ/mol and -2457.00 kJ/mo. When there are genistein, ΣE required total increased to -2393.71 kJ/mol and -2393.86 kJ/mol, as seen in Figure 5.
Figure 5: Docking pose of NF-κB with MMP-2 or MMP-9 promoter. The NF-κB represented as ribbon form and MMP-2 or MMP-9 promoter as helix model. Genistein has been bound to the transcription factor NF-κB, it will cause a decrease in the possibility of interaction between NF-κB by MMP-2 and MMP-9 gene promoter which is characterized by an increase in bond energy needed for the process of interaction.
Discussion
In order to induce endometriosis, we use adenomyosis tissue as implants. As defined, adenomyosis is a condition in which the inner lining of the uterus (the endometrium) breaks through the muscle wall of the uterus (the myometrium). Therefore, in this model, we induce the development of endometriosis of the entire endometrial tissue that has a high proliferative phenotype. The main finding of this study that adenomyosis tissue implants can lead to the development of endometriosis. Genistein was able to reduces the size of endometriosis lesion in the peritoneum of endometriosis mice. This finding indicates that genistein may inhibit the proliferation or induce apoptosis of endometriosis cells. Our finding confirmed previous studies that genistein also able to induce regression of endometriosis lesion [24]. The initial phase of endometriosis pathogenesis involves the extracellular matrix (ECM) breakdown, which regulate by matrix metalloproteinases (MMPs). In addition, the peritoneal invasion of endometrial tissue is also thought to be dependent on MMPs. Alteration of MMP-9 and MMP-2 is an important factor in the development of endometriosis [32-34]. In this study, the level of MMP-2 and MMP-9 was significantly higher in endometriosis group compared with the untreated group (P<0.05). This finding indicates that endometriosis induces upregulation of MMP production. The mechanism of upregulation of MMP maybe due to protease-activated receptor 1. Previous studies found that the proliferation of endometriosis cells happened via protease-activated receptor 1 (PAR1). PAR1 downstream signaling induces expression of matrix metalloproteinases [8].
Our study found that genistein inhibit MMP-2 and MMP-9 expression in a mice model of endometriosis. All doses genistein significantly prevented EM-induced increase in MMP-2 level, to reach the level in the control group. Previous studies showed that genistein exhibited a dose-dependent inhibition of expression of MMP-2 due to down-regulates the transcription and translation of genes [35,36]. Interestingly, the second dose of genistein able to significantly decrease the MMP-9 level, to reach a similar level with the control group. Various in vitro studies on HeLa cells and breast cancer cell line also proved that the inhibitory effects of genistein on the expression of MMP-9 were found in a very narrow range of doses [37-39]. NF-κB and AP-1 is a key regulator in the promoter region of MMP-9, while the AP-1 was not found in the promoter region of MMP-2. These differences underlie the mechanism of inhibition of MMP-2 expression by genistein occur through inhibition of the transcription factor NF-κB, are dose-dependent. Meanwhile, given that there are two regulators for the expression of MMP-9, allegedly only the inhibition of NF-κB and AP-1 against.
Our above hypothesis were supported by our in silico prediction that the genistein has been bound to the transcription factor NF-κB, it will cause a decrease in the possibility of interaction between NF-κB by MMP-2 and MMP-9 gene promoter which is characterized by an increase in bond energy needed for the process of interaction. In the analysis of the composition of chemical bonds are formed, genistein can reduce the stability of the bond between NF-κB with MMP gene promoters because the bond formed between the two is getting a little bit, so that it could be expected to decrease the activity of the transcription factor NF-κB.
In conclusion, our in silico and in vivo study show that genistein prohibits the increase in MMP-2 and MMP-9 in a mice model of endometriosis. Although, the resolution of endometriosis lesion cannot complete, this may provide a natural therapy for attenuating the alteration of MMPs found in endometriosis.
Declaration of Interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article.
References
- Mahmood TA, Templeton(1991) A Prevalence and genesis of endometriosis. Hum Reprod 6: 544-549.
- Olive DL, Schwartz LB (1993) Endometriosis N Engl. J Med 328:1759-1769.
- Guo SW, Wang Y (2006) Sources of heterogeneities in estimating the prevalence of endometriosis in infertile and previously fertile women.FertilSteril86:1584-1595.
- Guo SW, Wang Y (2006) The prevalence of endometriosis in women with chronic pelvic painGynecolObstet Invest62:121-130.
- Farquhar CM(2000) Extracts from the clinical evidence Endometriosis. BMJ 320:1449-1452.
- Abbas S, Ihle P, Koster I, Schubert I(2012) Prevalence and incidence of diagnosed endometriosis and risk of endometriosis in patients with endometriosis-related symptoms findings from a statutory health insurance-based cohort in Germany. Eur J ObstetGynecolReprodBiol 160:79-83.
- Janssen EB, Rijkers AC, Hoppenbrouwers K (2013) Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain a systematic review. Hum Reprod Update 19:570-582.
- Jiang QY, Wu RJ (2012) Growth mechanisms of endometriotic cells in implanted places a review.GynecolEndocrinol 28:562-567.
- D’Hooghe TM, Debrock S (2002) Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum Reprod Update 8:84-88.
- Donnez J, Wayembergh M, Casanas-Roux F (1987) Effect on ovulation of surgically induced endometriosis in rabbits. GynecolObstet Invest 24:131-137.
- Vernon MW, Wilson EA(1985) Studies on the surgical induction of endometriosis in the rat. FertilSteril 44:684-694.
- Sharpe TimmsKL (2002) Using rats as a research model for the study of endometriosis. ProcNatlAcadSci USA 955:318-327.
- Cummings AM, Metcalf JL (1995) Induction of endometriosis in mice a new model sensitive to estrogen. ReprodToxicol 9:233-238.
- Fang Z, Yang S, Lydon JP (2004) Intact progesterone receptors are essential to counteract the proliferative effect of estradiol in a genetically engineered mouse model of endometriosis. FertilSteril 82:673-678.
- Efstathiou JA, Sampson DA, Levine Z (2005)Nonsteroidal anti-inflammatory drugs differentially suppress endometriosis in a murine model. FertilSteril 83:171-181.
- Malik RK, Parsons JT (1996) Integrin-mediated signaling in normal and malignant cells a role of protein tyrosine kinases. BiochimBiophysActa 1287: 73-76.
- Seynaeve CM, Stetler-Stevenson M, Sebers S (1993) Cell cycle arrest and growth inhibition by the protein kinase antagonist UCN-01 in human breast carcinoma cells. Cancer Res 53: 2081-2086.
- Matrisian LM (1992) The matrix-degrading metalloproteinases.Bioessays 14:455-463.
- Saare M, Lamp M, Kaart T (2010)Polymorphisms in MMP-2 and MMP-9 promoter regions are associated with endometriosis. FertilSterilSep 94:1560-1563.
- Shaco-Levy R, Sharabi S, Benharroch D (2008) Matrix metalloproteinases 2 and 9 E-cadherin and beta-catenin expression in endometriosis low-grade endometrial carcinoma and non-neoplastic eutopic endometrium Eur. J ObstetGynecolReprodBiolAug 139:226-232.
- Qian Y, Guan T, Huang M (2012)Neuroprotection by the soy isoflavonegenistein via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. NeurochemInt 60:759-767.
- Gossner G, Choi M, Tan L (2007)Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol Oncol 105:23-30.
- Tsuchiya M, Miura T, Hanaoka T (2007) Effect of soy isoflavones on endometriosis: interaction with estrogen receptor 2 gene polymprphismEpidemiology 18:402-408.
- Yavuz E, Oktem M, Esinler I (2007)Genistein causes regression of endometriotic implants in the rat model. FertilSteril 88:1129-1134.
- Yu XP, Mi MT, Zhu JD (2004) Effect of genistein on expression of angiogenesis related factors in HER-2/neu-overexpressing breast cancer cells.37:251-253.
- Barnes S, Peterson TG, Coward L (1995) Rationale for the use genistein-containing soy matrics in chemoprevention trials for breast and prostate cancer. J Cell Biocherm 59 :181-187.
- Sutrisno S, Wulandari RRCL, Sulistyowati DWW (2015) Effect of genistein on proinflammatory cytokines and estrogen receptor-b in mice model of endometriosisAsian Pacific. J Reprod 4:96-99.
- Arnold K, Bordoli L, Kopp J (2006) The SWISS-MODEL workspace a web-based environment for protein structure homology modelling. Bioinformatics 22:195-201.
- van Dijk M, Bonvin AMJJ (2008) 3D-DART a DNA structure modelling serverNucleic Acids Res 37: 235-239.
- Macindoe G, Mavridis L, Venkatraman V (2010) HexServer an FFT-based protein docking server powered by graphics processors. Nucleic Acids Res 38:445-449.
- Luscombe NM, Laskowski RA, Thornton JM (1997) NUCPLOT a program to generate schematic diagrams of protein nucleic acid interactionsNucleic Acids Res 25:4940-4945.
- Bruner KL, Matrisian LM, Rodgers WH (1997) Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest 99:2851-2857.
- Bruner KL, Eisenberg E, Gorstein F (1999) Progesterone and transforming growth factor-beta coordinately regulate suppression of endometrial matrix metalloproteinases in a model of experimental endometriosis Steroids 64:648-653.
- Collette T, Maheux R, Mailloux J (2006) Increased expression of matrix metalloproteinase-9 in the eutopic endometrial tissue of women with endometriosis. Hum Reprod21:3059-3067.
- Su SJ, Yeh TM, Chuang WJ (2005) The novel targets for anti-angiogenesis of genistein on human cancer cells. BiochemPharmacol 69:307-318.
- Li Y, Sarkar FH (2002) Down-regulation of invasion and angiogenesis-related genes identified by cDNA microarray analysis of PC3 prostate cancer cells treated with genisteinCancer Lett 186:157-164.
- Li Y, Bhuiyan M, Sarkar FH (1999) Induction of apoptosis and inhibition of c-erb-B in MDA-MB-435 cells by genisteinInt J Oncol 15:525-533.
- Shao ZM, Wu J, Shen ZZ, Barsky SH (1998)Genistein exerts multiple suppressive effects on human breast carcinoma cellsCancer Res 58:4851-4857.
- Ling H, Yang H, Tan SH (2010) 6-Shogaol an active constituent of ginger inhibits breast cancer cell invasion by reducing matrix metalloproteinase 9 expression via blockade of nuclear factor kB activation British. J Pharmacol 161:1763-1777.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences