Circadian Rhythm of Oestradiol: Impact on the Bone Metabolism of Adult Males
Wijetilleka S, Mon A, Khan M, Joseph F, Robinson A, Vora JP and Fraser WD
Wijetilleka S1*, Mon A2, Khan M1, Joseph F3, Robinson A4, Vora JP1 and Fraser WD5
1Department of Endocrinology, Royal Liverpool University Hospital, Liverpool, United Kingdom
2Department of Endocrinology, University Hospital Aintree, Liverpool, United Kingdom
3Department of Diabetes and Endocrinology, Countess of Chester NHS Foundation Trust, Chester
4Department of Clinical Biochemistry, Royal Liverpool University Hospital, Liverpool, United Kingdom
5Department of Medicine, Bob Champion Research and Education Building, University of East Anglia, Norwich Research Park, Norwich, Norfolk
- *Corresponding Author:
- Wijetilleka S
Department of Endocrinology
Royal Liverpool University Hospital
Liverpool, United Kingdom
Tel: +44 151 706 2000
Fax: +44 151 706 2000
Email: Sajini.Wijetilleka@nhs.net
Received date: July 26, 2016; Accepted date: August 23, 2016; Published date: August 26, 2016
Citation: Wijetilleka S, Mon A, Khan M, Joseph F, Robinson A, et al. (2016) Circadian Rhythm of Oestradiol: Impact on the Bone Metabolism of Adult Males. J Clin Mol Endocrinol 1:20. doi: 10.21767/2572-5432.100018
Copyright: © 2016 Wijetilleka S, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Few studies have examined the variation in oestradiol with respect to age and circadian rhythm and the subsequent effects on BMD. Aim: Demonstrate the presence or absence of a circadian rhythm for oestrodial in older men and the integral role of concerted circadian rhythms of several factors including parathyroid hormone (PTH) in regulating biochemical markers of bone resorption and formation. Examine whether concentrations of both circulating total and bioavailable oestrogen in men differ with age and BMD. Design: Males were recruited: young men with normal BMD, older men with normal BMD and older men with osteoporosis. Methods: Subjects were hospitalized for a 25-hour period. Blood samples were obtained every 30 minutes. Hormone analysis results were plotted and reviewed. Results: Both total and bioavailable oestradiol concentrations were significantly lower in the older men than the young men (Total oestradiol: 34.5±4.4 pmol/L vs. 49.0±6.5 pmol/L, p<0.0001; Bioavailable oestradiol 16.7±2.2 pmol/L vs. 26.3±3.6 pmol/L, p<0.0001). Bioavailable oestrogen rhythm mirrored that of total estrogen. Conclusion: Both age groups with normal BMD display circadian rhythmicity with respect to circulating and bioavailable oestradiol. Younger men have increased mean total and bioavailable oestrogen concentrations and later acrophase compared to older counterparts. In older men with low BMD, total circulating oestrogen was not significantly different compared to age-matched older men with normal BMD; bioavailable oestrogen was significantly lower. Total oestrogen demonstrated a concerted circadian rhythm in all 3 groups, but bioavailable oestrogen did not demonstrate circadian rhythmicity in older men with decreased BMD.
Introduction
Circulating free and total oestradiol concentrations correlate with bone mineral density (BMD) in men [1-5]. Oestradiol has been shown to regulate both bone formation and resorption in normal elderly men [1]. Circulating total oestradiol decreases with age in men [1,2] and is associated with increased circulating osteoprotegerin concentrations. Treatment with low dose oestrogen has been shown to decrease markers of bone resorption in elderly men [1].
We have recently demonstrated a circadian rhythm for osteoprotegerin in older men1 and the integral role of concerted circadian rhythms of several factors including parathyroid hormone (PTH) in regulating biochemical markers of bone resorption and formation which also demonstrate circadian rhythms is established [1,2].
It has been reported that circulating total oestradiol demonstrates a diurnal rhythm in men [1,2] but not by others [3,4]. There have also been conflicting reports with some studies demonstrating a morning peak as of Bodenheimer [1] and others demonstrating a late afternoon peak of oestradiol of Juneja. The circadian rhythm of oestradiol may be a contributory factor to bone metabolism in men over and above absolute concentrations.
About 20% of oestrogen in men is produced in the testes [1] and the remainder is either derived from the peripheral aromatisation of testosterone (around 60%) or by the reversible conversion of circulating oestrone (20%) [2].
Oestradiol circulates in the blood in one of three forms: bound to sex hormone binding globulin (SHBG) (37%); bound to albumin (61%); and a small percentage is unbound or free (2-3%) [1]. The albumin bound fraction of oestradiol is bound with low affinity and easily dissociated. This albumin bound oestradiol together with the free fraction is considered to be biologically active: it can potentially cross the cell membrane, and is termed bioavailable [1].
Free or bioavailable oestradiol has previously been calculated using total oestradiol and SHBG concentrations and an assumed association constant for albumin and SHBG in a validated algorithm [1-3] or by adapting the method described for measuring non-SHBG-bound testosterone first described by Tremblay and Dube. This adaptation has been validated as a satisfactory estimate of non-SHBG-bound estradiol [1].
To help explain the link between oestrogen and bone metabolism in men we investigated the differences in circulating concentrations as well as circadian rhythms of total and bioavailable oestradiol in young healthy control men with normal bone mineral density (BMD), older men with normal BMD and older men with low BMD.
Subjects and Methods
Subjects
Healthy subjects were recruited from hospital personnel and a database of volunteers willing to participate in medical research. Subjects with low BMD had been identified in a community osteoporosis screening program. Nineteen subjects were recruited for the study; six young healthy men with normal BMD, eight older men with normal BMD and five older men with osteoporosis.
All volunteers had BMD measured by dual X-ray Absorptiometry (DXA) using a Prodigy Oracle Fan-Beam bone densitometer (GE Medical Systems, Wisconsin, USA). T-scores were calculated against a reference population of UK subjects 20-39 years of age. All subjects had circulating total testosterone concentrations in the reference range. Subjects with diabetes, ischaemic heart disease, heart failure, renal disease, cancer, chronic illness, vertebral fracture or any disease or medication such as corticosteroids, affecting the skeleton were excluded.
Subjects were excluded if they were on calcium and vitamin D supplements or had previous exposure to bisphosphonate therapy. Body weight and body mass index were well matched in all three groups. Baseline characteristics and T-scores are listed in (Table 1). The local ethics research committee approved the study, and all patients provided written informed consent before recruitment.
| Healthy young men | Elderly men with normal BMD | Elderly men with osteoporosis | |
|---|---|---|---|
| Age years | 27.3±4.6 | 70.5±2.1 | 75.0±2.9 |
| BMI | 25.3±2.3 | 26.5±2.8 | 24.7±4.4 |
| LumbarSpine T score |
0.08±1.36 | 0.98±1.65 | -1.96±1.25 |
| Femoral Neck T score |
-0.25±0.74 | -0.28±0.79 | -2.94±0.35 |
| IGF 1 ug/L | 160±42 | 112±24 | 72±13 |
| CTX ug/L | 0.59±0.22 | 0.34±0.22 | 0.69±0.20 |
| P1NP ug/L | 83±37 µg/L | 44±19 | 60±13 |
| Testosterone nmol/L | 22.2±3.0 | 15.1±4.0 | 20.1±8.3 |
| SHBG nmol/L | 10.5±4.9 | 12.8±4.7 | 24.6±8.4 |
| 24 hour total oestradiolpmol/L | 49.0±6.5 | 36.8±3.9 | 34.5±4.4 |
| Bioavailable oestradiolpmol/L | 26.3±3.6 | 16.7±2.2 | 12.5±1.8 |
CTX: type I collagen C-telopeptides;
P1NP : amino-terminal procollagenpropeptide 1
Table 1: Demographic characteristics of the three groups [Mean ±SEM].
Subjects
Volunteers were hospitalized at 1300 h for a 25 hour period. Blood samples were obtained every 30 minutes from 1400 - 1400 hr via an indwelling venous cannula inserted in the antecubital fossa at the time of admission. Each time 7 mL blood was collected and the samples were immediately centrifuged, and the plasma was aliquoted and stored at -80°C before analysis. Subjects remained recumbent during 2300-0800 h and slept during this period. Each patient was served preset standardized plated hospital meals at 0800, 1200 hours and 1800 hours. The serving sizes and combinations of foods contained recommended daily allowances of all nutrients including calcium and phosphate. Patients were provided with one and a half litres of water and encouraged to drink at fairly frequent intervals to maintain hydration. Urine samples were collected at 3-hourly intervals between 1400-2300 h, overnight from 2300-0800 h and 3- hourly from 0800-1400 h with a 24 hour urine volume estimation to assess fluid balance. No significant variability was observed in the hydration of individuals.
Biochemical Measurements
Total oestradiol was measured by radioimmunoassay using the Diagnostic Products (DPC) double antibody sensitive oestradiol kit method (Diagnostic Products Ltd, Llanberris, North Wales). Each subject’s samples were assayed in the same batch. Intra-assay and between assay variation was 6% and 9% at 20 pmol/L and 7% and 9% at 70 pmol/L respectively. Bioavailable oestradiol was measured using minor modifications of the Trembley and Dube assay [1]. Briefly, serum is stripped of steroids using activated charcoal before incubation at 37 degree centigrade with [3] H-labelled oestradiol. SHBG is precipitated from the serum using saturated ammonium sulphate, and the percentage free and albumin bound oestradiol was determined from the radioactivity in the supernatant. The concentration of bioavailable oestradiol was then calculated from the total oestradiol. Intra-assay and between assay precision was 5% and 4% at 10 pmol/L and 3% and 3.5% at 50 pmol/L respectively.
To test the precipitation technique and ensure that all the SHBG was precipitated by the ammonium sulphate, SHBG concentrations were assayed in the supernatant after the addition of saturated ammonium sulphate to the serum. The 10 samples used had SHBG concentrations ranging between 16–106 nmol/L, with one sample from a pregnant woman whose SHBG concentration was >200 nmol/L. SHBG was undetectable in all the supernatant from all of the sera used, indicating that the ammonium sulphate precipitates all the SHBG.
In our laboratory between-assay precision has been established at 7.1% at 22.7 pmol/L and 8.6% at 81 pmol/L. Comparing our measured results with calculated values by Deming regression analysis revealed an equation of y =1.4x + 3.89 with r2 =1.00. As bioavailable oestradiol is the biologically active component, measurements using direct assay techniques may better reflect the role of estrogens in metabolism.
Statistical Analysis
Individual and population mean cosinor analysis was used first to confirm circadian rhythmicity and determine the circadian rhythm variables of total oestradiol and bioavailable oestradiol using CHRONOLAB 3.0 (Universdade de Vigo, Vigo, Spain), a software package for analyzing biological time series by least squares estimation. The software thus provides the following circadian rhythm variables: 1) midline estimate statistic of rhythm (MESOR), defined as the rhythm-adjusted mean or the average value of the rhythmic function fitted to the data; 2) amplitude, defined as half the extent of rhythmic change in a cycle approximated by the fitted cosine curve (difference between the maximum and MESOR of the fitted curve); and 3) acrophase, defined as the lag between a defined reference time (1400 h of the first day in our study when the fitted period is 24 h) and time of peak value of the crest time in the cosine curve fitted to the data. A p value for the rejection of the zero-amplitude (no rhythm) assumption is also determined for each individual series and for the group. The method used by the program allows analysis of hybrid data (time series sampled from a group of subjects, each represented by an individual series). Given k individual series, the program fits the same linear model with m different frequencies (harmonics or not from one fundamental period) to each series. This fit provides estimations for 2m + 1 parameters, namely, the amplitude and acrophase of each component, as well as the rhythm-adjusted mean. The population parameter estimates are based on the means of estimates obtained from individuals in the sample. The confidence intervals depend on the variability among individual parameter estimates. The variance-covariance matrix is then estimated on the basis of the sample covariances. Confidence intervals for the rhythm-adjusted mean, as well as for the amplitude-acrophase pair, of each component is then computed using the estimated covariance matrix. The p-values for testing the zero-amplitude assumption for each component, as well as for the global model, are finally derived using those confidence intervals and the t and F distributions. Bingham’s test, developed for testing cosinor parameters and part of CHRONOLAB 3.0 software, was used to determine the significance of the differences of cosinorderived circadian rhythm parameters between subjects.
The differences between groups were determined using ANOVA for repeated measures taking into account the 49 measurements for each individual in all 3 groups. This method has been previously validated for similar comparisons. Repeated measures ANOVA assumes normally distributed errors, equal variances and sphericity. The Kolmogorov- Smirnov test was used to confirm normal distribution and Levenes’s test for equality of variances. Mauchly’s test indicated that the sphericity assumption was violated and degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε=0.62). The between-group comparisons of circadian parameters however was performed with a single value per individual in each group, using students t-test for unpaired data. This comparison is subject to type 2 error given the limited number of individuals in the study and the values of circadian parameters presented in (Table 2) are MESOR, amplitude and acrophase from the population mean cosinor analysis (CHRONOLAB 3) for the analytes in all 3 groups. Values are expressed as the mean ÃÆïÃâââ¬Å¡Ãâñ SEM. p<0.05 was considered significant.
| Groups | MESOR±SEM (pmol/L) |
Amplitude±SEM (pmol/L) |
Time at peak (hour) |
Rhythmicity (p value) |
|---|---|---|---|---|
| Bioavailable oestradiol | ||||
| Healthy young men (HYM group) | ||||
| HYM1 | 16.2±0.6 | 4.1±0.9 | 12:25 | <0.001* |
| HYM2 | 13.7±0.3 | 1.8±0.4 | 14:43 | <0.001* |
| HYM3 | 41.4±0.7 | 7.0±0.9 | 11:07 | <0.001* |
| HYM4 | 28.8±0.7 | 6.6±0.9 | 10:07 | <0.001* |
| HYM5 | 29.3±0.78 | 3.0±1.1 | 00:55 | 0.029* |
| HYM6 | 21.4±0.6 | 5.6±0.8 | 12:41 | <0.001* |
| Elderly men with Normal BMD (NBMD) | ||||
| NBMD1 | 20.2±1.0 | 2.8±1.4 | 7:36 | 0.157 |
| NMBD2 | 5.7±0.4 | 1.4±0.5 | 6:15 | 0.038* |
| NBMD3 | 19.2±0.4 | 2.0±0.5 | 8:00 | <0.001* |
| NBMD4 | 36.4±0.8 | 6.5±1.2 | 5:08 | <0.001* |
| NBMD5 | 15.1±0.7 | 1.5±0.9 | 9:50 | 0.275 |
| NBMD6 | 20.0±0.3 | 5.4±0.5 | 8:43 | <0.001* |
| NBMD7 | 8.7±0.3 | 1.630.4 | 9:24 | <0.001* |
| NBMD8 | 13.8±0.5 | 1.9±0.7 | 11:15 | 0.023* |
| Elderly men with Osteoporosis (OBMD) | ||||
| OBMD1 | 14.7±0.6 | 0.9±0.8 | 6:48 | 0.583 |
| OBMD2 | 13.7±0.3 | 0.6±0.5 | 5:31 | 0.467 |
| OBMD3 | 6.4±0.2 | 0.3±0.3 | 12:48 | 0.484 |
| OBMD4 | 15.7±0.4 | 1.3±0.6 | 11:31 | 0.100 |
| OBMD5 | 11.7±0.4 | 0.4±0.6 | 00:03 | 0.799 |
| Groups | MESOR±SEM (pmol/L) |
Amplitude±SEM (pmol/L) |
Time at peak (hour) |
Rhythmicity (p value) |
| Total oestradiol | ||||
| Healthy young men (HYM) | ||||
| HYM1 | 30.3±0.9 | 6.0±1.3 | 14:48 | <0.001* |
| HYM2 | 32.9±0.7 | 4.3±1.0 | 13:48 | <0.001* |
| HYM 3 | 76.5±1.1 | 14.0±1.5 | 11:36 | <0.001* |
| HYM4 | 61.4±1.4 | 15.8±2.0 | 9:51 | <0.001* |
| HYM5 | 54.2±1.38 | 3.4±1.9 | 11:27 | 0.233* |
| HYM6 | 31.6±0.9 | 9,0±1.2 | 14:55 | <0.001* |
| Older men with normal BMD (NBMD) | ||||
| NBMD1 | 21.4±0.6 | 3.4±0.8 | 9:40 | <0.001* |
| NBMD2 | 40.3±1.6 | 4.4±2.3 | 8:40 | 0.178 |
| NBMD3 | 31.3±0.8 | 4.9±1.2 | 12:03 | <0.001* |
| NBMD4 | 36.8±0.6 | 8.7±0.9 | 10:40 | <0.001* |
| NBMD5 | 77.6±1.3 | 14.5±1.8 | 8:40 | <0.001* |
| NBMD6 | 28.1±1.0 | 3.4±1.4 | 10:15 | 0.053 |
| NBMD7 | 10.0±0.8 | 1.7±1.1 | 13:43 | 0.275 |
| NBMD8 | 36.6±0.7 | 4.8±1.0 | 8:31 | <0.001* |
| Older men with Osteoporosis (OBMD) | ||||
| OBMD1 | 44.8±1.0 | 3.1±1.5 | 10:40 | 0.107 |
| OBMD2 | 29.6±0.9 | 2.5±1.3 | 10:24 | 0.166 |
| OBMD3 | 27.1±0.4 | 2.5±0.6 | 13:36 | <0.001* |
| OBMD4 | 49.9±1.0 | 7.2±1.4 | 10:12 | <0.001* |
| OBMD5 | 32.6±0.8 | 1.8±1.1 | 15:58 | 0.226 |
Table 2: Cosinor analysis of circadian rhythm parameters of bioavailable and total oestradiol.
Results
Young healthy men vs. older men with normal BMD
24 h mean concentrations: Both total and bioavailable oestradiol concentrations were significantly lower in the older men than the young men (Total oestradiol: 34.5±4.4 pmol/L vs. 49.0±6.5 pmol/L, p<0.0001; Bioavailable oestradiol 16.7± 2.2 pmol/L vs. 26.3±3.6 pmol/L, p<0.0001).
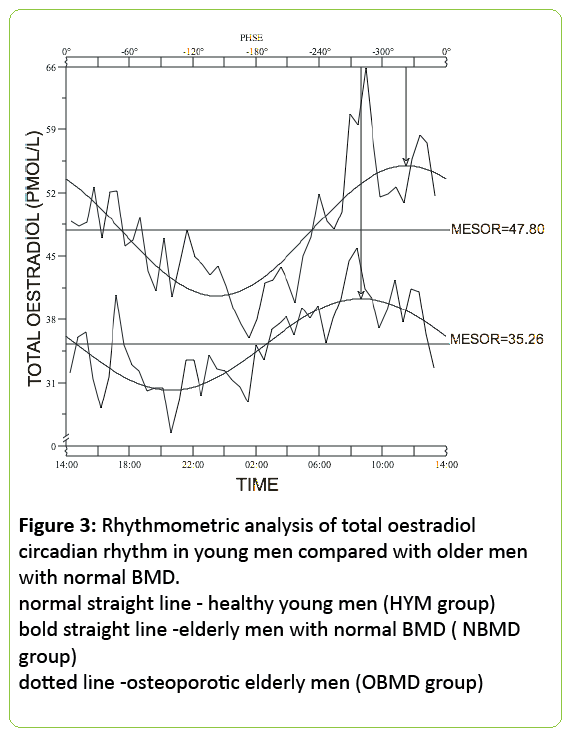
Circadian rhythm: Both total and bioavailable oestradiol demonstrated concerted circadian rhythmicity with a morning peak and night time nadir in all the young men and the older men with normal BMD (Figure 1).
Bioavailable oestrogen rhythm mirrored that of total estrogen. The group mean MESOR for total oestrogen for the young men was 47.8±7.8 pmol/L with an amplitude of 7.2±2.7 pmol/L and acrophase at 11:27 am.
The group mean MESOR for bioavailable oestrogen for the young men was 23.6±4.1 pmol/L with an amplitude of 4.2±1.0 pmol/L and acrophase at 11:15 am. The group mean MESOR for total oestrogen for the older men with normal BMD was 35.3±7.0 pmol/L with an amplitude of 5.1±1.5 pmol/L and acrophase at 8:31 am.
The group mean MESOR for bioavailable oestrogen for the older men with normal BMD was 16.8±3.4 pmol/L with an amplitude of 2.3±0.6 and acrophase at 7:40 am. The rhythmometric parameters for the individuals are listed in (Table 2). The MESORs for both total oestrogen and bioavailable oestrogen were significantly higher in the young men when compared with the older men with normal BMD (p<0.001).
With respect to IGF1, the group means MESOR for young healthy men was 160 ±42 mcg/L; older men with normal bone density had a group mean MESOR of 112±24 mcg/L. The older men with reduced BMD had a group mean MESOR of 72±13 mcg/L. CTX and PINP analysis showed that younger men and elderly men with reduced BMD had higher group mean MESORs than older men with normal BMD CTX 0.59±0.22 mcg/L and PINP 83±37 mcg/L for younger men compared to (CTX 0.69±0.2 mcg/L and PINP 60±13 mcg/L for older men with reduced BMD and CTX 0.34±0.22 mcg/L and PINP 44±19 mcg/L for older men with normal BMD.
Testosterone results showed a pattern consistent with this; group mean MESOR 22.2±3.0 mcg/L for younger men, 20.1±9.3 mcg/L for older men with reduced BMD and 15.1±4.0 mcg/L for older men with normal BMD. This is reflected in the SHBG results - group mean MESOR 10.5±4.9 mcg/L for younger men, 24.6±8.4 mcg/L for older men with reduced BMD and 12.8±4.0 mcg/L for older men with normal BMD.
Older men with normal BMD versus older men with low BMD
Older men with a reduced BMD had a higher mean testosterone and SHBG result compared to those with normal bone density. Their PINP and CTX levels were also significantly higher than that of counterparts with normal bone density, with levels similar to those of younger men.
IGF-1 levels were significantly greater in older men with normal bone density (group mean MESOR 112±24 mcg/L compared to those with reduced bone density 72±13 mcg/L.
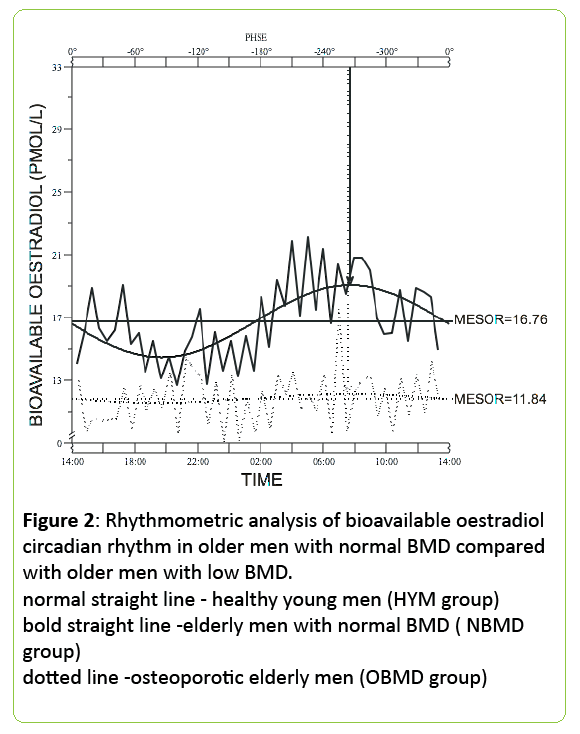
Higher SHBG levels indicate that not all the testosterone present in the serum is available for bone mineralisation thus inferring a lower free testosterone level which may result in lower BMD (Figure 2).
Figure 2: Rhythmometric analysis of bioavailable oestradiol circadian rhythm in older men with normal BMD compared with older men with low BMD. normal straight line - healthy young men (HYM group) bold straight line -elderly men with normal BMD ( NBMD group) dotted line -osteoporotic elderly men (OBMD group)
24 h mean concentrations
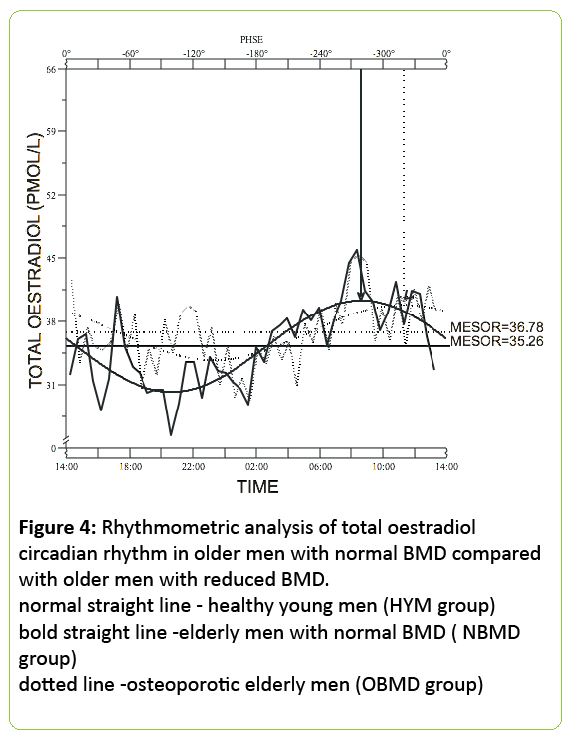
24h mean total oestradiol was similar in both groups of older men (36.8±3.9 pmol/L for normal BMD subjects vs. 34.5±4.4pmol/L, for low BMD - p<0.0001) but the circulating bioavailable oestradiol concentration was significantly lower in the older men with low BMD (12.5±1.8 pmol/L) when compared with the men with normal BMD (16.7±2.2 pmol/L; p<0.0001) (Figures 3 and 4).
Figure 4: Rhythmometric analysis of total oestradiol circadian rhythm in older men with normal BMD compared with older men with reduced BMD. normal straight line - healthy young men (HYM group) bold straight line -elderly men with normal BMD ( NBMD group) dotted line -osteoporotic elderly men (OBMD group)
Discussion
Our findings demonstrate that concentrations of both circulating total and bioavailable oestrogen in men differ with age. Whilst both age groups with normal BMD display circadian rhythmicity with respect to circulating and bioavailable oestradiol, younger men have higher mean total and bioavailable oestrogen concentrations compared to older counterparts. Younger men have a later acrophase compared to older subjects. In older men with low BMD, total circulating oestrogen was not significantly different when compared to age-matched older men with normal BMD, but bioavailable oestrogen was significantly lower. Total oestrogen demonstrated a concerted circadian rhythm in all 3 groups, but bioavailable oestrogen did not demonstrate circadian rhythmicity in older men with decreased BMD. Concentrations of IGF-1 were higher in younger men compared with older counterparts, more so between the young healthy group (160±42 mcg/L) and the elderly group with decreased BMD (72± 13 mcg/L). This suggests that the IGF-1 axis may contribute towards the maintenance of BMD as patients with higher levels of BMD have a higher level of IGF-1, inferring that somatotrophin levels rise with BMD. The mean concentrations of CTX although lower were not statistically different between younger men and the older group with reduced BMD (0.59 mcg/L vs. 0.69 mcg/L). The mean CTX for older men with normal BMD was 0.34 mcg/L. PINP results follow a different pattern with mean concentrations of 83 mcg/L for younger men, 60 mcg/L for older men with reduced BMD and 44 mcg/L for older men with normal bone density. This indicates that overall bone remodeling rates are highest in younger men and lowest in older men with normal BMD and that “uncoupling” is present in men with decreased BMD with the highest rate of bone resorption and the lowest rate of bone formation. The combination of the difference in resorption and formation markers suggests a role for regulating hormones acting on the osteoclast and osteoblast.
The regulation of bone metabolism and maintenance of BMD in men is complex. The GH and IGF-1 axis [5-7], PTH5-7 and testosterone [5-7] have been shown to contribute. Even though androgens are the major sex steroids in men, their predominance in regulating male skeletal remodelling has been questioned as there is evidence to suggest that circulating oestrogen has a role in regulating bone mineral metabolism and bone density in men [1-6]. Evidence to support the role of oestrogen comes in part from studies in adult men, rendered temporarily hypogonadal and then administered aromatase inhibitors. The increase in bone resorption markers after induction of the hypogonadal state was almost completely prevented by oestradiol, but not by testosterone therapy alone, indicating that the increase in bone resorption was due primarily to oestrogen, not testosterone loss. It has also been suggested that a threshold level of bioavailable oestradiol is needed to prevent bone loss, and that with ageing, a percentage of elderly men begin to fall below this level. Aromatase activity and hence circulating estrogen can differ as a result of polymorphisms at the human aromatase (CYP19) gene, as well as tissue dependent modulation of aromatase; it is worth considering whether aromatase activity differs with bone density as older men with lower BMD have higher testosterone levels but similar total and bioavailable oestradiol levels to subjects of normal BMD of the same age [5-12]. Some subjects have almost no circulating oestrogen due to the polymorphism inherited and require transdermal oestrogen therapy in order to maintain ageappropriate skeletal development, bone density and sperm atogenesis. These differences become more pronounced in older men [5] and may account for the difference in bioavailable oestrogen observed in the 2 groups of older men in our study. Our observation of the lower bioavailable oestrogen concentration in the men with low BMD also supports the role of oestrogen in maintaining bone density in men. Recent population based studies [6-24] have also suggested that SHBG may also be an independent predictor for bone loss in men. Our finding of the presence of a lower bioavailable oestradiol concentration in the presence of similar total oestradiol concentrations in the men with low BMD when compared with the men with normal BMD would be in keeping with the previously demonstrated association of a raised SHBG and low BMD. It is likely however that a higher SHBG results in a lower availability of biologically active oestrogen resulting in a lack of maintenance of bone mineral metabolism and BMD.
The morning peak and nocturnal nadir in total and bioavailable oestrogen in the young healthy men and older men with normal BMD is in keeping with previous reports of Bodenheimer and Carlsen [1]. The absence of a concerted circadian rhythm in bioavailable oestrogen in the older men with low BMD suggests that as with other endocrine regulators of bone metabolism, rhythmicity of bioavailable oestrogen may be important, over and above circulating concentrations alone. When compared with the previously reported rhythm of circulating testosterone [25], both total and bioavailable oestradiol rhythms lag behind those of testosterone by approximately 2-4 hours possibly reflecting the peripheral conversion of testosterone to oestrogen, which is the predominant source of circulating oestrogen in men.
It has previously been shown that markers of bone resorption demonstrate an entrained circadian rhythmicity with a nocturnal peak in both men and women. Alterations in this rhythmicity have been studied in more detail in postmenopausal women who are oestrogen deficient and may be mediated at least in part by altered PTH and osteoprotegerin rhythms. The circadian rhythm of oestrogen we observed in the young men and the older men with normal BMD appear to be opposite to the previously described rhythm of bone resorption markers. It is possible that the higher nocturnal concentrations of resorption markers may be a result of the lack of oestrogen restraint on osteoclasts. Future studies would require the assessment of bone turnover markers and oestrogen in older men with low BMD, most particularly the effects of oestradiol on altering PTH responsiveness and the dual variables of a modified/deficient PTH rhythm with altered circulating oestradiol rhythms in order to enhance our understanding.
We believe our findings support a significant role for circulating oestrogen in regulating bone turnover in men. Our study demonstrates that concentrations of both circulating total and bioavailable oestrogen in men differ with age. Whilst both age groups with normal BMD display circadian rhythmicity with respect to circulating and bioavailable oestradiol, younger men have increased mean total and bioavailable oestrogen concentrations compared to older counterparts. Younger men have a later acrophase compared to older subjects. In older men with low BMD, total circulating oestrogen was not significantly different when compared to age-matched older men with normal BMD, but bioavailable oestrogen was significantly lower. Total oestrogen demonstrated a concerted circadian rhythm in all 3 groups, but bioavailable oestrogen did not demonstrate circadian rhythmicity in older men with decreased BMD. IGF-1 levels appear to the a significant factor in the maintainance of bone density across age groups, with reduced BMD subjects demonstrating lower levels than those with normal BMD. In conclusion, our study adds a further piece of information to understanding the complex pathophysiology of the development of osteoporosis in men.
References
- Slemenda CW (1997) Sex steroids and bone mass in older men: positive associations with serum estrogens and negative associations with androgens. J Clin Invest 100:1755-1759.
- Greendale GA, Edelstein S, Barrett-Connor E (1997) Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 12: 1833-1843.
- Khosla S, Melton LJ, Atkinson EJ, O'Fallon WM, Klee GG, et al. (1998) Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J ClinEndocrinolMetab 83: 2266-2274.
- Ongphiphadhanakul B (1998) Serum oestradiol and oestrogen-receptor gene polymorphism are associated with bone mineral density independently of serum testosterone in normal males. ClinEndocrinol 49:803-809.
- Center JR, Nguyen TV, Sambrook PN, Eisman JA (1999) Hormonal and biochemical parameters in the determination of osteoporosis in elderly men. J ClinEndocrinolMetab 84: 3626-3635.
- Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, et al. (2000) Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106: 1553-1560.
- Riggs BL, Khosla S, Melton LJ. (1998) A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res 13: 763-773.
- Szulc P, Munoz F, Claustrat B, Garnero P, Marchand F, et al. (2001) Bioavailable estradiol may be an important determinant of osteoporosis in men: the MINOS study. J ClinEndocrinolMetab 86: 192-199.
- Taxel P, Raisz LG(1997) The effect of estrogen therapy on older men with low bone mass. J Bone Miner Res 12:S353.
- Joseph F, Chan BY, Durham BH, Ahmad AM, Vinjamuri S, et al. (2007) The circadian rhythm of osteoprotegerin and its association with parathyroid hormone secretion. J ClinEndocrinolMetab 92: 3230-3238.
- Ahmad AM, Hopkins MT, Fraser WD, Ooi CG, Durham BH, et al. (2003) Parathyroid hormone secretory pattern, circulating activity, and effect on bone turnover in adult growth hormone deficiency. Bone 32:170-179.
- Ahmad AM, Thomas J, Clewes A, et al. (2003) Effects of growth hormone replacement on parathyroid hormone sensitivity and bone mineral metabolism. J ClinEndocrinolMetab88:2860-2868.
- Carlsen E, Olsson C, Petersen JH, Andersson AM, Skakkebaek NE (1999) Diurnal rhythm in serum levels of inhibin B in normal men: relation to testicular steroids and gonadotropins. J ClinEndocrinolMetab 84: 1664-1669.
- Juneja HS, Karanth S, Dutt A (1991) Diurnal variations and temporal coupling of bioactive and immunoactive luteinizing hormone, prolactin, testosterone and 17-beta-estradiol in adult men. Horm Res 35:89-94.
- Nankin HR, Pinto R, Fan DF (1975) Daytime titers of testosterone, LH, estrone, estradiol, and testosterone-binding protein: acute effects of LH and LH-releasing hormone in men. J ClinEndocrinolMetab41:271-281.
- Murono EP, Nankin HR, Lin T, Osterman J (1982) The aging Leydig cell V. Diurnal rhythms in aged men. ActaEndocrinol 99: 619-623.
- Bodenheimer S, Winter JS, Faiman C (1973) Diurnal rhythms of serum gonadotropins, testosterone, estradiol and cortisol in blind men. J ClinEndocrinolMetab 37: 472-475.
- Sayed Y, Taxel P (2003) The use of estrogen therapy in men. CurrOpinPharmacol 3: 650-654.
- de Ronde W, Pols HA, van Leeuwen JP, de Jong FH (2003) The importance of oestrogens in males. ClinEndocrinol58: 529-542.
- Pardridge WM (1986) Serum bioavailability of sex steroid hormones. ClinEndocrinolMetab 15: 259-278.
- Rinaldi S, Geay A, Dechaud H, Biessy C (2002). Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev 11:1065-1071.
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H (1982) Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 16: 801-810.
- Vermeulen A, Goemaere S, Kaufman JM (1999) Testosterone, body composition and aging. J Endocrinol Invest 22: 110-116.
- Karim R, Hodis HN, Stanczyk FZ, Lobo RA, Mack WJ (2008) Relationship between serum levels of sex hormones and progression of subclinical atherosclerosis in postmenopausal women. J ClinEndocrinolMetab 93: 131-138.
- Szulc P, Munoz F, Claustrat (2001) Bioavailable estradiol may be an important determinant of osteoporosis in men. J ClinEndocrinolMetab 86:192-199.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences