Application of Nano-biotechnology for Improvement in Therapeutic Approaches for the Treatment of Diabetes
Mukhopadhyay A, Prosenjit M
DOI10.21767/2572-5432.100047
Antara Mukhopadhyay and Prosenjit Mondal*
School of Basic Sciences, BioX, Indian Institute of Technology, Mandi, India
- *Corresponding Author:
- Prosenjit Mondal
School of Basic Sciences
BioX, Indian Institute of Technology Mandi
HP 175005, India
Tel: 01905237924
E-mail: prosenjit@iitmandi.ac.in
Received date: April 19, 2018; Accepted date: May 09, 2018; Published date: May 16, 2018
Citation: Mukhopadhyay A, Prosenjit M (2018) Application of Nano-biotechnology for Improvement in Therapeutic Approaches for the Treatment of Diabetes. J Clin Mol Endocrinol 3:2
Copyright: © 2018 Prosenjit M, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Diabetes mellitus is a chronic metabolic disorder which results in elevated blood glucose. It can be broadly classified as Type 1 and Type 2, depending on the reason for the high blood glucose levels. In Type 1 diabetes enough insulin is not produced by pancreatic beta cells, whereas in Type 2 the body's cells poorly respond to the presence of insulin. Nanotechnology holds a great deal of promise in diabetes management for efficient drug delivery, generation of smart drugs which only activate when needed. Anti-diabetes therapy is significantly transformed by using nanotechnology in many ways like in delivery of therapeutic molecules, fabrication of nanosensors to monitor dynamic changes in blood glucose level, and development of smart imaging molecule to visualise and quantify beta cells etc. This review article explores the way in which nanotechnology could be used to effectively treat, and possibly cure, diabetes. We anticipate in the long run, nanotechnology will clearly open up many routes to treatments and participate in understanding and finding a cure that would improve the lives of all people affected by diabetes.
Keywords
Type 2 diabetes; Nano biotechnology; Therapeutics; Pancreatic beta cell; Insulin secretion
Introduction
The incidence of diabetes has reached epidemic status in world; International Diabetes Federation estimates that there are approximately 422 million individuals with Type 2 Diabetes (T2DM) in world and as predicted by The World Health Organization, the number is expected to reach a staggering 600 million by 2030 [1].
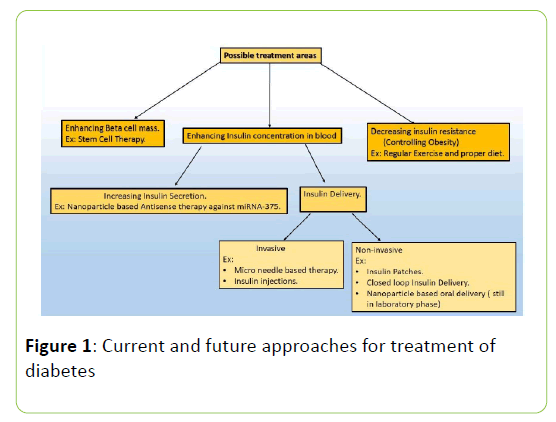
Diabetes Mellitus is a chronic metabolic disorder that is the result of elevated blood glucose levels because of the abnormalities associated with blood insulin levels. Insulin is a 51 amino acid long polypeptide chain which is secreted from pancreas. After a meal is taken, blood insulin level is elevated to cause the breakdown of glucose that is needed to provide energy to do necessary work in the body. Insulin maintains the blood glucose homeostasis by suppressing the glucagon secretion from pancreatic alpha cells, thereby enhancing the glucose intake in muscles [2]. But if somehow the insulin secretion or the mechanism of action of insulin is blocked, hyperglycaemic condition appears which may eventually lead to other malfunctions of the body such as high blood pressure, ketoacidosis, Proteinuria etc. Diabetes Mellitus is of two types: Type 1 diabetes and Type 2 diabetes. Another type of diabetes, gestational diabetes occurs during pregnancy because insulin signalling pathway in the placenta of pregnant women is defective [3] but the Type 1 and Type 2 diabetes is of main concern because they are chronic in nature. The immune cells of our body are responsible for attacking foreign particles. But sometimes due to defective immune cell maturation procedure or because of the presence of some specific biomarkers (in diseased condition) the immune cells attack body’s own cells causing destruction of cells and eventually leads to autoimmune disorders. A major example of this autoimmune disorder is Type 1 diabetes where body’s immune cells invade into the pancreatic beta cells to cause destruction of beta cell mass which leads to very small or no production of insulin. The Type 2 diabetes is also called Non-insulin dependent diabetes as it occurs because of the Insulin resistance in insulin sensitive tissues [4]. It is also caused because of the beta cell dysfunction at later stages of diabetes that eventually leads to the decrease of Insulin production [4]. Nano biotechnology is recent advancement in the field of biology and medicine which is in its growing phase. Nanoparticles exhibits much more efficient physicochemical properties than bulk because of the presence of unsatisfied bonds [5]. Nano biotechnology exploits the enhanced optical, catalytic, surface active properties and size dependent tunable properties of nanoparticle to detect, monitor and treat a disease [6]. In this review we represent the possible nano-biotechnology based therapeutic approaches to treat and monitor diabetes in a more effective way (Figure 1).
Nano-biotechnology-A brief introduction
Nanocarriers are the recent approaches to increase the therapeutic efficiency and bioavailability of drugs. Nanocarriers are used to increase the shelf life of any drug by preventing the drug from early degradation, rescue the drug from the uptake of reticuloendothelial system and increase the permeability of the drug through membranes. Nanocarriers are used for target specific delivery and hence bioavailability is high [7]. Some nanocarriers such as lipid derived nanoparticles are rapidly phagocytosed in liver cells and hence their circulation time is less [8]. Opsonin proteins (ex: complement proteins, immunoglobulins etc.) identifies the foreign nature of the nanoparticle and coats it either with high affinity (hard corona) or low affinity (soft corona). Phagocytic cells such as neutrophils, macrophages identifies the opsonin proteins which then binds with opsonin receptors on the phagocytic cells and gets internalized by those cells followed by degradation in lysosome [9,10]. Opsonin proteins bind strongly to hydrophobic surfaces of nanoparticles using weak interactions such as vander waals forces, electrostatic interactions etc [11,12]. Therefore to lower the clearance rate of nanoparticles, hydrophilic molecules such as PEG is used as they will not allow the opsonin proteins to sit on the nanoparticle because of the repulsive forces. PEG is covalently attached with nanoparticle surfaces which generate stealth property in nanoparticles which cannot be recognised by phagocytic cells because opsonins will be unable to sit on the surface of nanoparticle [13].
There are three generations of nanocarrier improvement. First generation involved only the drug-nanocarrier conjugate which had very less circulation time in blood because of the opsonisation. Second generation eliminated the problem associated with the phagocytosis by making the nanoparticle stealth by PEGlylation and improved the target specific delivery by introducing specific target specific ligands. Whereas third generation involves the modification of second generation nanocarrier based delivery system by introducing stimuli responsive characters [10]
Nanomaterials used for drug delivery include Carbon Nanotubes, Liposome, Gold nanorods, nanoshells etc. Targeting agents for target specific drug delivery include monoclonal antibody, Aptamers, Vitamins, Carbohydrates and Peptides etc [14,7]. Some Stimuli Responsive systems include heat sensitive (liopsomoes with leucine zipper motif), light sensitive (Gold nanocages, nanorods), magnetism responsive (magneto liposomes), Ultrasound sensitive (Perflurocarbon), Electro responsive (Polypyrrole), pH sensitive (polybase and poly acid ionisable groups are attached), Redox Sensitive, Self-regulated system (Phenylboronic acid that senses change in glucose concentration in blood) etc [15].
Nanoparticle internalization involves phagocytic or nonphagocytic pathways such as clathrin, caveolae or macropinocytosis based methods. Opsonisation is involved in phagocytic based internalization procedure whereas clathrin and caveolae mediated internalization involves GTPase dependent internalization where some ligands are attached to the surface on the carrier that can bind to specific receptors on the cell surface for internalization. Examples of ligands used for receptor mediated endocytosis include low density lipoproteins, epidermal growth factor, transferrin etc. for clathrin mediated endocytosis and cholesterol, albumin, folic acid etc. for caveolae mediated
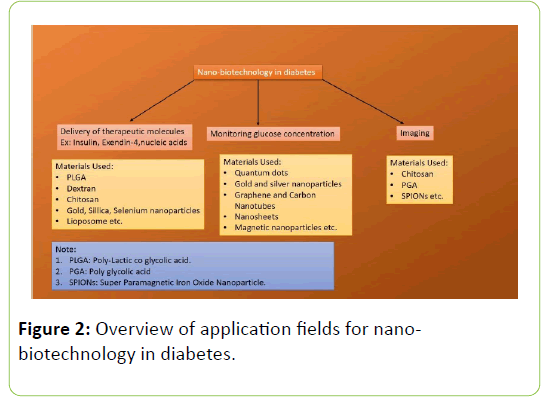
Endocytosis [9,10] Phagocytosis and clathrin mediated endocytosis ends up in lysosome. Therefore they are not preferred mode of delivery but if the nanocarrier is positively charged, it can get out of the lysosome showing “Proton Sponge Effect” [10,12]. Hence caveosome is preferred mode of delivery in many cases to avoid lysosomal degradation (Figure 2). Caveolae mediated endocytosis is also preferred in adipose tissues as caveolae is abundant there [9,12].
Application of nanotechnology in diabetes
Delivery of therapeutic molecules
The main disadvantage of Insulin delivery is that it cannot be delivered orally. Insulin is a peptide in nature and hence it is subjected to the first pass metabolism. The circulation time of insulin becomes very low due to protease degradation in the gastrointestinal tract and hence bioavailability of the orally delivered Insulin is very low [16]. Hence several methods are applied for effective drug delivery. Insulin, loaded in biotinylated liposomes showed improved receptor mediated absorption, increased bioavailability and lowered the hyperglycemic condition [17]. Insulin loaded with bile salt containing liposomes also showed enhanced absorption and permeation through the membranes [18] PLGA nanoparticles loaded with insulin, under pH stimulus showed improved bioavailability when administered orally [19,20] Another pH sensitive oral insulin delivery cascade involves chitosan-PGA-DTPA nanocarrier conjugate which protects the drug from protease degradation [21]. As Chitosan does not possess any cytotoxicity, chitosan itself and its various forms are used for controlled release of orally delivered insulin [22]. Increased penetration of insulin at the mucous region can be obtained using chitosan, modified chitosan such as trimethyl chitosan-cysteine nanoparticle or Chitosan reduced gold nanoparticle carrier [23-25]. Dextran sulphate, acrylic polymers, hyaluronic acid based polymers are also used to make nanoparticles for the delivery of insulin orally [26]. A pH sensitive chitosan microgel was designed with nano-capsule coated glucose oxidase to release insulin in a controlled manner under hyperglycemic condition (the pH change was sensed as glucose was converted to gluconic acid) [27]. Besides Insulin, Fatty acid modified Exendin-4 can also be delivered through pulmonary route using chitosan coated PLGA nanoparticle to reduce the hyperglycemic effect of type-2 diabetes that showed lower clearance rate of drug-carrier conjugate in lungs [28]. It was reported that chitosan coated PLGA nanoparticle was engulfed by clathrin and macropinocytosis whereas only PLGA coated nanoparticle was internalized by caveolin [29]. Exendin-4 can be conjugated with positively charged low molecular weight chitosan to increase the half-life of the drug and to produce effective hypoglycemic effect [30]. Zincoxide and silver nanoparticles increased glukokinase acitivity and expression of GLUT-2 receptors in diabetic rats [31]. Diabetic ulcers are of major concern in diabetes and hence antioxidants such as EGCG (Epigallocatechin gallate) are used for treatment. Hence the low bioavailability associated with EGCG can be reduced by gasinjection of gold nanoparticles with EGCG [32]. Selenium nanoparticles alone or when mixed with insulin decreased the glucose 6 phosphatase activity and therefore decreased endogenous glucose production [33]. Silver-gold core shell nanoparticle reduced the fat content to alleviate the diabetic inflammation in diabetic rats [34,35]. Mesoporous Sillica nanoparticles are used for delivery of glucose responsive release of insulin and also cAMP to induce production of insulin from cells by activating calcium channels [36]. Exenatide was put into pH sensitive cross-linked polymers of alginate and hyaluronate and was subjected to treat Type-2 diabetes in mice that was able to reduce the glucose level to normal level rapidly [37]. Insulin and exendin-4 both can be loaded in chitosan-PGA nanoparticle and was useful to treat hyperglycemia in type-2 diabetic rats [38]. Beside insulin and exendin-4, RNA complimentary to miRNA-375 that causes the down regulation of insulin secretion is delivered to the pancreatic beta cell line MIN6 cells using novel liposome beta-MEND which is reported to be internalized by clathrin mediated pathway [39]. Triazole modified alginate nanoparticles were reported to be used as encapsulation for beta cells derived from human stem cell to control hyperglycemia in diabetes [40].
Nanoparticle conjugated biosensors
Change in glucose concentration in blood is of major concern in diabetes and therefore it is important to monitor the glucose concentration regularly. Nanoparticle was used in third generation electrochemical biosensors to increase the electron transfer rate [41]. Electrochemical biosensors utilizes Glucose oxidase, Glucose dehydrogenase, hexokinase etc. to determine the glucose concentration. Among these enzymes, glucose oxidase is used commonly because of its low cost and relative high stability. Glucose oxidase (GOD) which contains FAD group as cofactor converts the glucose to gluconic acid and gets FAD reduced to FADH2 followed by transfer of the electrons to mediator to form FAD again. The steps are as follows:
GOD (FAD)+glucose=Gluconic Acid+FADH2
FADH2+ O2= FAD+H2O2
The H2O2 is then measured in the electrode
Based on the use of mediators glucose biosensors evolved in three generations. The first generation included the use of oxygen as mediator and the response was dependent on oxygen concentration in the solution but the efficiency was low as normal oxygen concentration is lower than blood glucose concentration. This obstacle was improved in second generation using artificial mediators in the place of oxygen for electron transfer. Artificial mediators included ferricyanide, ferrocene derivatives, phenoxazine etc. Besides there are many contaminations (ex: drugs) which can give false responses when reacted with oxygen but this problem can be controlled in several ways. Coating the electrode with semipermeable membranes, use of artificial peroxidases such as Prussian blue nanoparticles can control the false responses [42,43]. Third generation eliminated the use of any mediator and glucose oxidase was directly attached to the electrode which increased the electron transfer rate by many fold. Examples include the immobilization of glucose oxidase (FAD)-Au nanoparticle onto Au electrode using thiol linkers [44]. After that there are many advancements in nanomaterial based glucose sensing. Nanomaterials include Au, Ag nanoparticle based sensor, CNT and graphene based sensor, Nanoparticle array, quantum dots etc [45]. Quantum dots can be used instead of fluorophores as they exhibit almost no photo-bleaching property. For example, glucose oxidase and CdTe quantum dots can be assembled to sense glucose concentration [46]. To substitute the flurophores in optical sensors, quantum dots can be used but they are toxic in nature [27]. Surface Plasmon Resonance is important characteristic of gold and silver nanoparticle which changes upon particle aggregation [47,48]. This feature is used to sense glucose concentration using dextran functionalized Au nanoparticle, glucose and saccharide binding Concanavalin-A (Con-A) that can bind to dextran and glucose both but binds to glucose with higher affinity. Therefore observing the change in SPR spectra upon addition of glucose (because of the separation of nanoparticle aggregates that formed due to multiple binding capacity of Con-A with dextran when glucose was absent) can be used in glucose concentration monitoring. The same can be achieved using boronic acid modified Ag nanoparticle also [49]. CNT based glucose biosensors are group of sensors which improves the efficiency because the CNTs possess very high electron shuffling rate due to large length to diameter ratio. For example, FAD can be covalently immobilized to the end of SWCNT (Single walled carbon nanotubes) followed by the attachment of glucose oxidase to FAD [50]. SWCNT also exhibit the emission of light in the NIR region when excited with light and thus is of great importance for monitoring glucose concentration continuously in vivo because of their no photo bleaching property. SWCNTs can be modified either with glucose binding proteins or Phenyl boronic acid which causes change in emission spectra of fluorescence as quenching happens when concentration of glucose changes [51]. CNT FET and CNT fibres are also used as sensors because of their ability to detect change in resistance with high sensitivity and very high catalytic efficiency due to large surface area respectively. Besides Enzymatic biosensors, non-enzymatic biosensors were made using CNT which was modified using Nickel, Copper etc. Good electric and mechanical properties of graphene make them useful for application in sensor technology. In both enzymatic and non-enzymatic biosensors graphene can be used to achieve high sensitivity and low detection limit [52] SWCNTs can also be used to detect glucose concentration in saliva. The device contained SWCNT which was physically adsorbed onto Platinum electrode followed by assembly of chitosan and gold nanoparticle where glucose oxidase was assembled [53]. In diseased condition several volatile organic compounds become abundant in breath and hence they can serve as useful biomarkers to detect a disease as in case of diabetes acetone concentration increases in breathe for lack of the insulin. An FET based (modified with indium nitride) gas sensor as well as ZnO nano sheets, Zr loaded WO3 pure and TixSn1-x02 was reported to have achieved detection of acetone at very low ppm level which can be of useful importance in the detection of diabetes [54,55]. Thin walled SnO2 fibres (made by electrospinning) were loaded with catalytic Pt nanoparticle which can sense the acetone concentration in breath samples [56]. Besides these, enzymes can be immobilized onto magnetic nanoparticle or platinum nanowire for glucose sensing [45]. Sweat based method of glucose concentration monitoring measures the glucose concentration with the help of a disposable patch that collects the sweat and deliver drug with a micro needle at certain glucose concentration. This disposable patch can be made of gold doped graphene and the glucose concentration can be monitored wirelessly using cell phone [57,58]. Prussian blue nanoparticle based amperometric sensors can be used in tattoos for non-invasive glucose monitoring [59].
Theranostics and imaging
Theranostics involves both the therapy and imaging using nanoparticle loaded with drug molecule and imaging agents [60]. In Type 1 diabetes the beta cell mass is already destroyed because of the infiltration of lymphocytes and other immune cells. In case of Type 2 diabetes there is slow destruction of beta cell mass because of the formation of ROS after long term metformin treatment and stress upon beta cell mass due to increased level of glucose in blood and insulin resistance in insulin sensitive tissues. Hence the quantification and regeneration of beta cell mass is very important to diagnose the diabetic patient and initiate proper treatment [61]. Regeneration can be achieved using stem cell therapy either by using embryonic stem cells [61] or adipose tissue derived stem cells [62]. If there is a rapid degradation of pancreatic beta cell mass and insulin secretion is much lower than normal, Stem cell therapy can be used to regenerate the beta cell mass to eradicate the problem from root though much more advancement in stem cell regeneration is needed. Though the pancreatic islets are investigated thoroughly in vitro, several complications lie during imaging of pancreatic beta cell mass invivo.
The first and foremost challenge includes the low distribution of endocrine cell mass in pancreas and finding proper markers that are associated with only Pancreatic beta cells to achieve targeted and specific imaging [63]. Possible imaging methods include Positron Emission Tomography, Single Photon Emission Computed Tomography (SPECT), MRI, CT etc. Imaging probes include antibody specific to TMEM-27, targeting ligands for PSANCAM, GLP-1 receptor, SUR-1 receptor etc [64]. In a study using rats, Exendin-4 loaded in I123 labelled chitosan-PGA nanoparticle was able to lower the glucose concentration in a pH sensitive way and the distribution of the drug in gastrointestinal tract was monitored using SPECT/CT scanner [65]. The beta cell targeting efficiency of exendin-4 was studied by loading exendin-4 in PEG modified super paramagnetic iron oxide molecule using Prussian blue staining and quantum dotexendin- 4 conjugate in INS-1 cell lines. The distribution profile in mice was studied using MRI [66]. Exendin-4 was loaded in PEG modified iron oxide magnetic nanoparticle to image the accumulation profile of the drug to target tissue using MRI. It was seen that beside receptor mediated uptake of the nanoparticle, passive targeting was also involved for the leaky structure in endothelium [67]. Macrophage inflammation in beta cell is the major issue in type-1 diabetes and therefore it is important to track the insulitis process. Dextran coated MRI sensitive magnetic nanoparticle or a super paramagnetic iron oxide MRI probe coupled with fluorophore can be used to study the inflammation by tracking the engulfment of the nanoparticle by the macrophages [68-71]. Superparamagnetic iron oxide nanaoparticles with dextran modifications were used to deliver fluorescent dyes for imaging the transplanted islets in diabetic mice using both MRI and fluorescent imaging [72].
Discussion
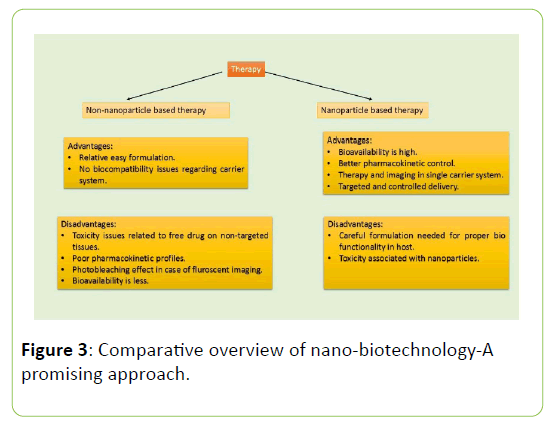
Insulin injection, proper diet and exercise can be used as treatment of diabetes but as of now, there is no cure for both the types of diabetes i.e Type 1 and Type 2 diabetes. Type 1 and Type 2 diabetes are diagnosed in children and adults respectively but in both the cases, blood glucose level is measured to check the hyperglycemic condition. There are several tests which confirms the diabetic stage. Fasting Plasma Glucose Test is used to measure the blood glucose level under fasting condition that is to have only water for at least 8 hours. A1C test is done to check the glycated haemoglobin. In RPG test, there is no need of fasting and the blood sugar level can be measured at any point of time. OGTT involves sensing of the impairment of glucose tolerance [73]. For Type 1 diabetes, insulin injection or insulin pump is the only solution till date [74]. As of Type 2 diabetes, there are several approaches to manage and monitor the disease (Figure 3).
Some possible treatments include use of drugs such as Metformin which is biguanide molecule which reduces the endogeneous glucose production by suppressing the production of cAMP which is key effector molecule in glucagon signalling [75], Sulphonylureas such as glibenclamide binds to SUR-1 receptors in pancreatic beta cells to increase the insulin secretion [76], GLP-1 receptors agonists that increase the insulin secretion, lowers the rate of gastric emptying and increase the glucose uptake [77] SGLT2 inhibitors, newest anti diabetic drugs make use of the inhibitors of Sodium Glucose Co-transporters. SGLT is involved in reabsorption of glucose in Kidney. Thereby inhibition of SGLT2 can mitigate the hyperglycemic condition in blood [78]. At first, diet, exercise, metformin etc. are prescribed for the treatment of Type 2 diabetes but at the later stages when blood sugar level is beyond control regular Insulin therapy is the only option left for possible treatment [79] Insulin can be delivered using insulin injections, pens, inhalers, pumps, etc [80]. There are typically two types of Insulin therapy present to maintain the basal insulin level. Long acting insulin has increased shelf life that can be present in blood for up to 24 hours 79 and fast acting Insulin which is taken just before the meals to elevate blood insulin level to help in glucose breakdown [81]. Insulin Pumps are subcutaneously given to Type 1 diabetic patients in forms of patches so that fast insulin can be released slowly [82]. The automatic Insulin Pumps works under partial closed loop system reduces the effect of hypoglycaemia [82]. Hypoglycemia can be caused because of elevated levels of insulin and can result in weakness, vision problems, unconsciousness etc [83,84]. Insulin patches and injections are often associated with patient’s irritation and other side effects. Besides these effects, if Insulin is not released in controlled manner there will be hypoglycemic condition which can lead to several other complications [84]. The GLP-1 agonist therapy also include several complications such as irritation at the site of injection, dizziness etc [85].
Conventional Glucose sensing includes electrochemical (amperometric), optical (fluorescence, Raman Spectroscopy etc. are used) biosensors, glucometer, microneedle, etc [86]. Electrochemical biosensors use mainly glucose oxidase to measure glucose concentration as mentioned earlier. Besides electrochemical sensors, decrease in quenching and increase in fluorescence intensity of labelled dextran and Energy transfer between labelled Con-A and dextran with the increase of glucose concentration can also be used to monitor glucose [87,88]. To reduce the inefficiency associated with enzymes, non-enzymatic biosensors using boronic acid which is based on Photo-induced Energy Transfer mechanism can be a good substitute [89,86]. Recently optical (fluorophore labelled carbohydrate receptor) and electrochemical sensors were coupled to achieve orthogonal redundancy with minimally invasive technique to measure glucose concentration in Type 1 diabetes persons. Although this system achieved high accuracy with glucose concentration monitoring, the optical sensing component needs to be improved so that it overcomes hurdles related to the adverse environment in a body [90]. A recent invitro study postulated non-invasive glucose monitoring using NIR spectra for different concentrations of glucose solution but further experiments are needed to improve the sensor response [91]. Glucose sensors are also coupled with insulin pumps to provide closed loop continuous glucose monitoring that can act as artificial pancreas [92,93] During the past decade closed-loop insulin delivery increased the efficiency of treatment of hypoglycemia and reduced the error associated with human intervention but still there is a need of improvement in terms of long term stability and energy efficiency [94].
In a study using animal models, NIR fluorescent probes were designed to specifically target GLP-1 agonist receptor in pancreatic beta cell mass [95]. Zn2+ is associated with mature insulin release from pancreatic beta cells as it is important to form insulin hexamer [96] and hence the release of zinc can be a useful marker to diagnose beta cell mass. Therefore a GLP-1 receptor targeted fluorescent marker (ZIMIR) was designed with exendin-4 to sense Zn2+ to detect functional beta cell mass [97,98]. But fluorophores are subjected to photo bleaching and hence alternate ways were needed [99]. Besides nanobiotechnology some possible alternate approach can be a bimodal imaging system using PET and NIR fluorophore, the use of a luminescent marker luciferase from Gaussia princeps to label c-peptide of insulin etc. but the later detection mechanism possess several disadvantages such as inability to detect the alterations in expression of insulin or the alteration of beta cell function due the presence of the reporter [100,101].
Nano-biotechnology has potential to be effective therapeutic options in diabetes treatment that can be used to overcome the problems associated with the present therapeutic approaches. As seen above the advancements included glucose sensing technology, oral insulin delivery and imaging technology but still there is a long way to go. The non-invasive breath based, minimally invasive wirelessly monitored sweat based, SWNT based sensors definitely project the advancements of glucosesensing technology but the sensitivity, selectivity, long term stability of glucose sensors when administered in vivo need to be improved [102,51]. Glucose sensors and oral insulin delivery are most abundant application field for Nano-technological advancements but still quantification of beta cell mass in vivo is a very difficult task either in conventional way or using nanoparticle [103]. Fluorophores are subjected to photobleaching and hence they are not applicable for continuous monitoring of beta cell mass which is necessary to diagnose the progression of the diabetes. Several nanoparticles are used to substitute fluorophores but complications arise due to their toxicity at higher concentration and long term application [104,105]. Besides imaging, further developments are needed for the delivery of the nanoparticle-drug conjugates because of the toxic effects of nanoparticles. Hence the design of a nanoparticle is a very important factor to be analysed.
As depicted above, there is a huge possibility of improvement in health care specifically in diabetes using nanoparticles if the structure, composition and chemical properties of the raw materials can be improved to make nanoparticle suitable for in vivo application specifically when applied to a human. We anticipate that among all possible nanoparticle based approaches, it is quite clear that chitosan comparatively possesses better biocompatibility in host as well as can protect the drug under harsh environment in gastrointestinal tract. Therefore, as per our view a PEG-chitosan nanoparticle modified with glucose oxidase and a pH actuator can be used for the oral delivery of insulin to control hyperglycemic condition for efficient and robust treatment of diabetes.
Acknowledgements
The authors are thankful to Director, Indian Institute of Technology, Mandi for his encouragement and financial support. PM acknowledges financial support from Department of Biotechnology, India (BT/PR22214/NNT/28/1267/2017) and Science and Engineering Research Board grant (ECR/ 2015/000165). The authors declare that there are no conflicts of interest associated with this review. All authors have approved the final version to be published.
References
- Kaveeshwar SA, Cornwall J (2014) The current state of diabetes mellitus in India. Australas Med J 7: 45-48.
- Aronoff SL, Berkowitz K, Shreiner B, Want L (2004) Glucose metabolism and regulation: Beyond insulin and glucagon. Diabetes Spectrum 17: 183-190.
- Kaaja R, Ronnemaa T (2008) Gestational diabetes: Pathogenesis and consequences to mother and offspring. Rev Diabet Stud 5:194-202.
- Kahn SE (2003) The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 46: 3-19.
- Roduner E (2006) Size matters: Why nanomaterials are different. Chemical Society Reviews 35: 583-592.
- Nam J, Won N, Bang J, Jin H, Park J et al. (2013) Surface engineering of inorganic nanoparticles for imaging and therapy. Adv Drug Deliv Rev 65: 622-648.
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, et al. (2007) Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology 2: 751-760.
- Petrak K (2018) Essential properties of drug targeting delivery systems. Drug Discov Today 10: 1667-1673.
- Hillaireau H, Couvreur P (2009) Nanocarrier’s entry into the cell: Relevance to drug delivery. Cell Mol Life Sci 66: 2873-2896.
- Verderio P, Avvakumova S, Alessio G, Bellini M, Colombo M, et al. (2014) Delivering colloidal nanoparticles to mammalian cells: A nano-bio interface perspective. Adv Healthc Mater 3: 957-976.
- Salmaso S, Caliceti P (2013) Stealth properties to improve therapeutic efficacy of drug nanocarriers. J Drug Deliv 374252: 1-20.
- Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, et al. (2009) Understanding biophysicochemical interactions at nano-biointerface. Nature materials 8: 543-557.
- Owens DE, Peppas NA (2006) Opsonization, biodistribution and pharmacokinetics of polymeric nanoparticles. Int J Pharm 307: 93-102.
- Sajja HK, East MP, Mao H, Wang AY, Nie S, et al. (2009) Development of multifunctional nanoparticles for targeted drug delivery and non-invasive imaging of therapeutic effect. Curr Drug Discov Technol 6:43-51.
- Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nature materials 12: 991-1003.
- Fonte P, Araújo F, Reis S, Sarmento B (2013) Oral insulin delivery: How far are we?. J Diabetes Sci Technol7: 520-531.
- Zhang X, Qi J, Lu Y, He W, Li X, et al. (2014) Biotinylated liposomes as potential carriers for the oral delivery of insulin. Nanomedicine 10: 167-176.
- Niu M, Tan Y, Guan P, Hovgaard L, Lu Y, et al. (2014) Enhanced oral absorption of insulin-loaded liposomes containing bile salts: A mechanistic study. Int J Pharm 460: 119-130.
- Wu ZM, Ling L, Zhou LY, Guo XD, Jiang W, et al. (2012) Novel preparation of PLGA/HP55 nanoparticles for oral insulin delivery. Nanoscale Res Lett 7: 1-8.
- Malathi S, Nandhakumar P, Pandiyan V, Webster TJ, Balasubramanian S (2015) Novel PLGA-based nanoparticles for the oral delivery of insulin. Int J Nanomedicine 10: 2207-2218.
- Fang-Yi S, Kun-Ju L, Sonaje K, Shiaw-Pyang W, Tzu-Chen Y, et al. (2012) Protease inhibition and absorption enhancement by functional nanoparticles for effective oral insulin delivery. Biomaterials 33: 2801-2811.
- Chaudhury A, Das S (2011) Recent advancements of chitosan-based nanoparticles for oral controlled delivery of insulin and other therapeutic agents. AAPS Pharm Sci Tech 12: 10-20.
- Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB (2007) Chitosan reduced gold nanoparticles for transmucosal delivery of insulin. Pharm Res 24: 1415-1426.
- Pan Y, Ying-jian L, Hui-ying Z, Jun-min Z, Hui X, et al. (2002) Bioadhesive polysaccharide in protein delivery system: Chitosan nanoparticles improve the intestinal absorption of insulin in-vivo. Int J Pharm 249: 139-147.
- Yin L, Ding J, He C, Cui L, Tang C, et al. (2009) Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials 30: 5691-5700.
- Fonte P, Araujo F, Silva C, Pereira C, Reis S, et al. (2015) Polymer-based nanoparticles for oral insulin delivery: Revisited approaches. Biotechnol Adv 33: 1342-1354.
- Gu Z, Dang TT, Ma M, Tang BC, Cheng H, et al. (2013) Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano 7: 6758-6766.
- Lee C, Ji Choi S, Kim I, Oh KT, Lee ES, et al. (2013) Long-acting inhalable chitosan-coated poly (lactic-co-glycolic acid) nanoparticles containing hydrophobically modified exendin-4 for treating type-2 diabetes. Int J Nanomedicine 8: 2975-2983.
- Wang M, Zhang Y, Feng J, Gu T, Dong Q, et al. (2013) Preparation, characterization and in vitro and in vivo investigation of chitosan-coated poly (d,I-lactide-co-glycolide) nanoparticles for intestinal delivery of exendin-4. Int J Nanomedicine 8: 1141-1154.
- Ahn S, In-Hyun L, Lee E, Kim H, Yong-Chul K, et al. (2013) Oral delivery of an anti-diabetic peptide drug via conjugation and complexation with low molecular weight chitosan. J Control Release 170: 226-232.
- Alkaladi A, Abdelazim AM, Afifi M (2014) Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int J Mol Sci 15: 2015-2023.
- Yi-Huei H, Chao-Yi C, Po-Jung C, Shan-Wen T, Chia-Nan C, et al. (2014) Gas injection of gold nanoparticles and anti-oxidants promotes diabetic wound healing. RSC Advances 4: 4656-4662.
- Al-Quraishy S, Dkhil MA, Moneim AEA (2015) Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int J Nanomedicine 10: 6741-6756.
- Shaheen TI, El-Naggar ME, Hussein JS, El-Bana M, Emara E, et al. (2016) Antidiabetic assessment; in-vivo study of gold and core-shell silver-gold nanoparticles on streptozotocin-induced diabetic rats. Biomed Pharmacother 83:865-875.
- Karthick V, Kumar VG, Dhas TS, Singaravelu G, Sadiq AM, et al. (2014) Effect of biologically synthesized gold nanoparticles on alloxan-induced diabetic rats-An in-vivo approach. Colloids Surf B Biointerfaces 122: 505-511.
- Zhao Y, Trewyn BG, Slowing II, Lin VSY (2009) Mesoporous sillica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic amp. J Am Chem Soc 131: 8398-8400.
- Zhang B, He D, Fan Y, Liu N, Chen Y (2014) Oral delivery of exenatide via microspheres prepared by cross-linking of alginate and hyaluronate. PLoS One 9: e86064.
- Chuang EY, Nguyen GT, Su FY, Lin KJ, Chen CT, et al. (2013) Combination therapy via oral co-administration of insulin and exendin-4-loaded nanoparticles to treat Type-2 diabetic rats undergoing OGTT. Biomaterials 34: 7994-8001.
- Yamada Y, Tabata M, Yasuzaki Y, Nomura M, Shibata A, et al. (2014) A nanocarrier system for the delivery of nucleic acids targeted to a pancreatic beta cell line. Biomaterials 35: 6430-6438.
- Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, et al. (2016) Long term Glycemic Control using polymer encapsulated, human stem-cell derived beta cells in immune-competent mice. Nat Med 22: 306-311.
- Thomas A, Heinemann L, Ramírez A, Zehe A (2016) Options for the development of noninvasive glucose monitoring: is nanotechnology an option to break the boundaries?. J Diabetes Sci Technol 10: 782-789.
- Chen C, Xie Q, Yang D, Xiao H, Fu Y, et al. (2013) Recent Advances in electrochemical glucose biosensors: A review. RSC Advances 3: 4473-4491.
- Chen C, Zhao XL, Li ZH, Zhu ZG, Qian SH, et al. (2017) Current and emerging technology for continuous glucose monitoring. Sensors (Basel) 17: e182.
- Xiao Y, Patolsky F, Katz E, Hainfeld JF, Willner I (2003) “Plugging into Enzymes”: Nanowiring of redox enzymes by a gold nanoparticle. Science 299: 1877-1881.
- Taguchi M, Ptitsyn A, McLamore ES, Claussen JC (2014) Nanomaterial-mediated biosensors for monitoring glucose. J Diabetes Sci Technol 8: 403-411.
- Li X, Zhou Y, Zheng Z, Yue X, Dai Z, et al. (2009) Glucose biosensor based on nanocomposite films of CdTe quantum dots and glucose oxidase. Langmuir 25: 6580-6586.
- Rechberger WP, Hhenau A, Leitner A, Krenn JR, Lamprecht B, et al. (2003) Optical properties of two interacting gold nanoparticles. Optical Communications 220: 137-141.
- Kelly KL, Coronado E, Zhao LL, Schatz GC (2003) The optical properties of metal nanoparticles: The influence of size, shape and dielectric environment. J Phys Chem B 107: 668-677.
- Aslan K, Zhang J, Lakowicz JR, Geddes CD (2004) Sachharide sensing using gold and silver nanoparticles-A review. J Fluoresc 14: 391-400.
- Balasubramanian K, Burghard M (2006) Biosensors based on carbon nanotubes. Anal Bioanal Chem 385: 452-468.
- Yum K, McNicholas TP, Mu B, Strano MS (2013) Single-walled carbon nanotube-based near-infrared optical glucose sensors toward in vivo continuous glucose monitoring. J Diabetes Sci Technol 7: 72-87.
- Zhu Z, Garcia-Gancedo L, Flewitt AJ, Xie H, Moussy F, et al. (2012) A critical review of glucose biosensors based on carbon nanomaterials: Carbon nanotubes and graphene. Sensors (Basel) 12: 5996-6022.
- Du Y, Zhang W, Wang ML (2016) Sensing of salivary glucose using nano-structured biosensors. Biosensors (Basel) 6: 10.
- Kao KW, Hsu MC, Chang YH, Gwo S, Yeh JA (2012) A sub-ppm acetone gas sensor for diabetes detection using 10 nm thick ultrathin InN FETs. Sensors (Basel) 12: 7157-7168.
- Fioravanti A, Morandi S, Carotta MC (2016) Chemoresistive gas sensors for sub-ppm acetone detection. Procedia Engineering 168: 485-488.
- Shin J, Seon-Jin C, Lee I, Doo-Young Y, Park CO, et al. (2013) Thin-wall assembled sno2 fibres functionalized by catalytic pt nanoparticles and their superior exhaled-breath-sensing properties for the diagnosis of diabetes. Advanced Functional Materials 23: 2357-2367.
- Lee H, Song C, Hong YS, Kim MS, Cho HR, et al. (2017) Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Science Advances 3: 1-8.
- Lee H, Choi TK, Lee YB, Cho HR, Ghaffari R, et al. (2016) A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nature nanotechnology 11: 566-574.
- Bandokar AJ, Jia W, YardÃÆââ¬Å¾ÃâñmcÃÆââ¬Å¾Ãâñ C, Wang X, Ramirez J, et al. (2015) Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal Chem 87: 394-398.
- Xie J, Lee S, Chen X (2010) Nanoparticle-based theranostic agents. Adv Drug Deliv Rev 62: 1064-1079.
- Meier JJ (2008) Beta cell mass in diabetes: A realistic therapeutic target. Diabetologia 51: 703-713.
- Nam JS, Kang HM, Kim J, Park S, Kim H, et al. (2014) Transplantation of Insulin secreting cells differentiated from human adipose tissue derived stem cells into Type 2 diabetes mice. Biochem Biophys Res Commun 443: 775-781.
- Leibiger IB, Caicedo A, Berggren PO (2012) Non-invasive in vivo imaging of pancreatic beta cell function and survival-A perspective. Acta Physiol (Oxf) 204: 178-185.
- Laurent D, Vinet L, Lamprianou S, Daval M, Filhoulaud G, et al. (2015) Pancreatic beta cell imaging in humans: Fiction or option?. Diabetes Obes Metab18: 6-15.
- Nguyen HN, Wey SP, Juang JH, Sonaje K, Ho YC, et al. (2011) The glucose-lowering potential of exendin-4 orally delivered via a pH-sensitive nanoparticle vehicle and effects on subsequent insulin secretion in-vivo. Biomaterials 32: 2673-2682.
- Zhang B, Yang B, Zhai C, Jiang B, Wu Y (2013) The role of exendin-4-conjugated superparamagnetic iron oxide nanoparticles in beta-cell-targeted MRI. Biomaterials 34: 5843-5852.
- Wang P, Yoo B, Yang J, Zhang X, Ross A, et al. (2014) GLP-1R-Targeting magnetic nanoparticle for pancreatic islet imaging. Diabetes 63: 1465-1474.
- Turvey SE, Swart E, Denis MC, Mahmood U, Benoist C, et al. (2005) Noninvasive imaging of pancreatic inflammation and its reversal in Type 1 diabetes. J Clin Invest 115: 2454-2461.
- Denis MC, Mahmood U, Benoist C, Mathis D, Weissleder R (2004) Imaging inflammation of the pancreatic islets in Type-1 diabetes. Proc Natl Acad Sci USA 101: 12634-12639.
- Gaglia JL, Guimaraes AR, Harisinghani M, Turvey SE, Jackson R, et al. (2004) Noninvasive imaging of pancreatic islet inflammation in Type 1A diabetes patients. J Clin Invest 121: 442-445.
- Gaglia JL, Harisinghani M, Aganj I, Wojtkiewicz GR, Hedgire S, et al. (2015) Noninvasive mapping of pancreatic inflammation in recent onset Type-1 diabetes patients. Proc Natl Acad Sci 112: 2139-2144.
- Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A (2005) In-vivo imaging of islet transplantation. Nat Med 12: 144-148.
- (2011) Standards of medical care in diabetes-2011. Diabetes Care 34: S11-S61.
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, et al. (2005) Diabetes control and complications trial/epidemology of diabetes inventions and complications (dcct/edic) study research group. intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353: 2643-2653.
- Miller RA, Chu Q, Xie J, Foretz M, Viollet B, et al.(2013) Biguanides suppress hepatic glucagon signalling by decreasing production of camp. Nature 494: 256-260.
- Sola D, Rossi L, Schianca GP, Maffioli P, Bigliocca M, et al. (2015) Sulphonylureas and their use in clinical practice. Arch Med Sci 11: 840-848.
- Kalra S, Baruah MP, Sahay RK, Unnikrishnan AG, Uppal S, et al. (2016) Glucagon like Peptide Receptor Agonists in the treatment of Type 2 Diabetes: Past, Present and Future. Indian J Endocrinol Metab 20: 254-267.
- Chao EC (2014) SGLT2 Inhibitors: A new mechanism for glycemic control. Clin Diabetes 32: 4-11.
- Philis-Tsimikas A (2013) Initiating basal insulin therapy in T2 diabetes: practical steps to optimize glycemic control. Am J Med 126. S21-S27.
- Al-Tabakha MM, Arida AI (2008) Recent Challenges in Insulin Delivery Systems: A review. Indian J Pharm Sci 70: 278-286.
- Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R (2015) Managing Diabetes with nanomedicine: Challenges and opportunities. Nat Rev Drug Discov 14: 45-57.
- McAdams BH1, Rizvi AA (2016) An overview of insulin pumps and glucose sensors for the generalist. J Clin Med 5: 5.
- Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, et al. (2013) Threshhold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 369: 224-232
- McCall AL (2012) Insulin therapy and hypoglycemia. Endocrinol Metab Clin North Am 41: 57-87.
- Prasad-Reddy L, Isaacs D (2015) A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context 4: 212283.
- Hui-Chen W, An-Rong L (2015) Recent Developments in blood glucose sensors. Journal of Food and Drug Analysis 23: 191-200.
- Russell RJ, Pishko MV, Gefrides CC, McShane MJ, Coté GL (1999) A fluorescence-based glucose biosensor using concanavalin A and dextran encapsulated in a poly (ethylene glycol) hydrogel. Anal Chem 71: 3126-3132.
- Pickup JC, Hussain F, Evans ND, Rolinski OJ, Birch DJ (2005) Fluorescence-based glucose sensors. Biosens Bioelectron 20: 2555-2565.
- Fang H, Kaur G, Wang B (2004) Progress in boronic acid-based fluorescent glucose sensors. J Fluoresc 14: 481-489.
- McAuley SA, Dang TT, Horsburgh JC, Bansal A, Ward GM, et al. (2016) Feasibility of an orthogonal redundant sensor incorporating optical plus redundant electrochemical glucose sensing. J Diabetes Sci Technol 10: 679-688.
- Haxha S, Jhoja J (2016) Optical based noninvasive glucose monitoring sensor prototype. IEEE Photonics Journal 8.
- Cengiz E, Sherr JL, Weinzimer SA, Tamborlane WV (2011) New-generation diabetes management: Glucose sensor-augmented insulin pump therapy. Expert Rev Med Devices 8: 449-458.
- Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, et al. (2010) Effectiveness of sensor-augmented insulin-pump therapy in Type 1 diabetes. N Engl J Med 363: 311-320.
- Battelino T, Omladic JS, Phillip M (2015) Closed loop insulin delivery in diabetes. Best Pract Res Clin Endocrinol Metab 29: 315-325.
- Reiner T, Kohler RH, Liew CW, Hill JA, Gaglia J, et al. (2010) Near-infrared fluorescent probe for imaging of pancreatic beta cells. Bioconjug Chem 21: 1362-1368.
- Li YV (2014) Zinc and insulin in pancreatic beta-cells. Endocrine 45:178-189.
- Li D, Huang Z, Chen S, Hu Z, Li WH (2015) GLP-1 Receptor Mediated Targeting of a Fluorescent Zn2+ sensor to Beta Cell Surface for imaging insulin/Zn2+ Release. Bioconjugate chemistry 26: 1443-1450.
- Li D, Chen S, Bellomo EA, Tarasov AI, Kaut C, et al. (2011) Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytic release (ZIMIR). Proc Natl Acad Sci USA 108: 21063-21068.
- Vaddiraju S, Burgess DJ, Tomas I, Jain FC, Papadimitrakopoulos F (2010) Technologies for continuous glucose monitoring: Current problems and future promises. J Diabetes Sci Technol 4: 1540-1562.
- Burns SM, Vetere A, Walpita D, Dancik V, Khodier C, et al. (2015) High-throughput luminiscent reporter of insulin secretion for discovering regulators of pancreatic beta-cell function. Cell Metab 21: 126-137.
- Brand C, Abdel-Atti D, Zhang Y, Carlin S, Clardy SM, et al. (2014) In Vivo Imaging of GLP-1R with a targeted bimodal pet/fluorescence imaging agent. Bioconjug Chem 25: 1323-1330.
- Novak MT, Reichert WM (2015) Modelling the physiological factors affecting glucose sensor function in vivo. J Diabetes Sci Technol. 9: 993-998.
- Arifin DR, Bulte JW (2011) Imaging of pancreatic islet cells. Diabetes Metab Res Rev 27: 761-766.
- Sajja HK, East MP, Mao H, Wang YA, Nie S, et al. (2009) Development of multifunctional nanoparticles for targeted drug delivery and non-invasive imaging of therapeutic effect. Curr Drug Discov Technol 6: 43-51.
- Xu C, Mu L, Roes I, Miranda-Nieves D, Nahrendorf M, et al. (2011) Nanoparticle-based monitoring of cell therapy. Nanotechnology 22: 494001.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences