Analysis of Expression of Luteal Genes during Induced Luteolysis and Rescue of Luteal Function in Bonnet Macaques and Pregnant Rats
Miya John, Priyanka Samji, Rahaman Khan H, Akshi Vashistha and Medhamurthy Rudraiah
Miya John, Priyanka Samji, Rahaman Khan H, Akshi Vashistha and Medhamurthy Rudraiah*
Department of Molecular Reproduction, Development and Genetics, Indian Institute of Science, Bangalore, India
- *Corresponding Author:
- Rudraiah M
Department of Molecular Reproduction
Development and Genetics
Indian Institute of Science
Bangalore,India
Tel: 91-80-22933460
Fax: 91-80-23600999
E-mail: rmm@mrdg.iisc.ernet.in
Received date: June 02, 2016; Accepted date: October 01, 2016; Published date: October 03, 2016
Citation: John M, Samji P, Khan HR, Vashistha A, Rudraiah M (2016) Analysis of Expression of Luteal Genes during Induced Luteolysis and Rescue of Luteal Function in Bonnet Macaques and Pregnant Rats. J Clin Mol Endocrinol 1:25. doi: 10.21767/2572-5432.100023
Copyright: © 2016 John M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Studies have been carried out to standardize induced luteolysis model systems utilizing female monkeys and pregnant rats. In monkeys, administration of a single injection of GnRH receptor antagonist, Cetrorelix (CET; 150 μg/kg BW s.c.,), on day 7 of the luteal phase led to profound decrease in serum progesterone (P4) concentration within 24 h (3.6 ± 1.1 vs 0.8 ± 0.2 ng/ml before and 24 h post CET, respectively p<0.05), and followed by premature onset of menses 96 h later. It was observed that a single intravenous injection of rhLH 24 h post CET treatment caused rapid stimulation of P4 secretion within 1 h that lasted up to 24 h. To elucidate the molecular mechanisms associated with brisk restoration of P4 levels, corpora lutea were collected from monkeys treated with VEH, CET, CET+PBS and CET+LH treatments for molecular analyses. Expression of StAR, P450scc, LDLR, 3βHSD and aromatase was decreased in CET and CET+1 h LH treated monkeys. In CET+8 h LH treated monkeys, expression of StAR, P450scc and aromatase was higher. Immunoblot analyses of phospho (p) StAR and total StAR indicated decreased pStAR and total StAR levels in CL of CET treated monkeys, but were higher after LH treatment. In pregnant rat studies, repeated injections of CET (150 μg/kg BW) were required to induce luteolysis. Similar to monkey studies, exogenous LH injection for 1 h in CET treated rats increased serum P4 levels. Expression of hmgcr, hmgcs, ldlr, p450ssc decreased, expression of 20α- hsd increased and pStAR expression was marginally higher after LH treatment. These findings provide evidence for a dynamic interplay of transcriptional and post translational changes in expression of luteal genes following endogenous LH inhibition and replacement studies, and the data further suggests resilience of the CL structure to recover quickly from the LH deprived state.
Keywords
Macaques; Pregnant rat; Cetrorelix; LH; Luteolysis; StAR; Steroidogenic genes
Introduction
A large body of evidence suggests that the development and maintenance of corpus luteum (CL) function requires the action of LH in several species [1-3]. In the past, animal models involving blockade of LH action and/or secretion by way of immunoneutralization [4] or removal of source of its secretion by way of hypothalamic lesion and institution of hypothalamic prosthesis experiments [5] have been utilized to examine the processes of luteolysis and rescue of CL function in higher primates. In the last two decades, use of gonadotropin releasing hormone receptor (GnRH R) antagonists for inhibition of LH secretion to study CL function has emerged as an important approach for studying induced luteal regression [6-10]. Several groups have elegantly demonstrated inhibition of progesterone (P4) secretion and induction of luteolysis following administration of GnRH R antagonist [11], and its reversal with pulsatile administration of exogenous human menopausal gonadotropin [12], incremental doses of human chorionic gonadotropin (hCG) [13] or recombinant human (rh) LH preparation [9]. However, at present, no suitable model exists for examining the role of LH in the regulation of CL function at cellular and molecular levels, and for delineation of signal transduction pathways employed by LH to mediate its multiple actions. The steroidogenic acute regulatory protein (StAR) has been demonstrated to play a critical role in tropic hormonestimulated steroidogenesis by facilitating cholesterol transfer to the inner mitochondrial membrane, the site of P450scc enzyme responsible for conversion of cholesterol into pregnenolone by [14]. In accordance with the observation of important role for StAR in steroidogenesis, our recent studies in the monkey CL revealed that its expression paralleled the circulating P4 levels during different functional status of CL confirming a tight association of StAR expression with the P4 levels [10].
In pregnant rats, several lines of evidence suggest that LH is crucial for maintenance of luteal function throughout gestation [1], however, no systematic studies have been reported to examine specific effects of inhibition of endogenous LH secretion on luteal steroidogenesis in pregnant rats. Moreover, it is not clear whether exogenous LH administration during luteolysis results in rescue of luteal function. With a view to examine luteal function and expression of luteal genes during induced luteolysis and LH replacement studies, several experiments were carried out in monkeys and rats with the following objectives: (i) to study the effects of administration of GnRH R antagonist treatment on the luteal function and to characterize early effects of withdrawal of LH on CL function in a systematic fashion, (ii) to examine the effects of administration of exogenous LH preparations on the rescue of luteal function during induced luteal regression with a view to develop model systems for delineating the signalling pathways associated with LH actions on structure and function of CL, and (iii) to describe the early effects of LH replacement in GnRH R antagonist treated animals on expression profiles of the various luteal genes.
Materials and Methods
Reagents
Oligonucleotide primers were synthesized by Sigma-Genosys, Bangalore, India. DyNAzyme™ II DNA polymerase (F-501L) and DyNAmo™ HS SYBR™ Green qPCR kit (#F410) were purchased from Finnzymes, Finland. Restriction enzymes, Moloney Murine Leukemia Virus (MMuLV) reverse transcriptase, and 100 bp DNA ladder were obtained from MBI Fermentas, Germany. GnRH R antagonist, Cetrorelix® (CET) was a kind gift from Asta Medica, Frankfurt, Germany. rhLH was a kind gift from Ares Serono, Aubonne, Switzerland and highly purified hLH was a kind gift from Dr. MR Sairam of Montreal University, Canada. Antibodies against StAR and phospho-StAR were kind gifts from Drs. DM Stocco and R King (Texas Tech University Health Sciences Center, Texas, USA), respectively. Antibody against β-actin (#A5441) and other reagents were purchased from Sigma Aldrich Co., Bangalore, India or sourced from local suppliers.
Animal Protocols, Blood Samples and CL Collection
Experimental protocols in monkeys and rats described here were approved by the Institutional Animal Ethics Committee of the Indian Institute of Science, Bangalore.
Experiments in Macaques (Macaca Radiata)
The general care and housing of monkeys at the Primate Research Laboratory, Indian Institute of Science, Bangalore have been described elsewhere [15,16]. The details of procedure employed for detecting the onset of estradiol (E2) surge has been described elsewhere [17]. Blood samples were collected either daily or at more frequent intervals until the time of CL retrieval or the onset of menses. For this study, 1 day after E2 peak was designated as day 1 of luteal phase. For analyses of expression of various genes and western blotting, corpora lutea were obtained from VEH, CET and CET+rhLH treated monkeys (see below) as described previously [18]. The CL was retrieved (see below for experimental details) from the ketamine hydrochloride (15 mg/kg BW) and/or pentobarbital sodium (8 mg/kg BW) anesthetized monkeys subjected to laparatomy under aseptic conditions. Immediately after collection, the CL was cut into 4-5 pieces and flash frozen in liquid nitrogen before storing at -70°C.
Example I: GnRH R antagonist-induced luteolysis
Experiments were carried out using CET, a third generation GnRH R antagonist, in the macaques during non-fertile menstrual cycles as reported previously [19,20]. In order to determine whether a single subcutaneous injection of CET would be adequate to induce luteolysis, a pilot study was carried out in which effects of a single injection of CET at a dose of 75 or 150 μg/kg BW was examined in monkeys (n=3 monkeys/dose) starting from day 7 of the luteal phase. Monkeys receiving 150 μg/kg BW showed fall in circulating P4 levels and exhibited onset of menses 96 h post CET treatment. Further, characterization of markers of luteolysis (expression of genes such as P450scc, StAR and inh-α in CL and other parameters) was carried out on monkeys receiving CET at a dose of 150 μg/kg BW at the end of 24hrs (see below) as reported previously [10]. Further experiments in monkeys have been carried out using 150 μg/kg BW dose of CET.
Example II: Effects of injection of exogenous LH on luteal function and expression of genes in CL of monkeys treated with a luteolytic dose of CET
In the previous experiment, a single injection of CET 150 μg/kg BW was able to induce luteolysis, pilot studies were carried out to determine the effect of administration of exogenous LH on luteal function. For this purpose, effects of intravenous injection of rhLH at doses of 10 and 20 IU/kg BW or 2 μg of highly purified hLH preparation were examined in monkeys treated with CET for 24 h. The results indicated that injection of rhLH (20 IU/kg BW) or hLH (2 μg) preparations elevated P4 levels in CET treated monkeys within 2 h to levels comparable to or higher than that observed in untreated monkeys on day 8 of luteal phase (data not shown). Group of monkeys (n=5), CET (150 μg/kg BW) was administered subcutaneous on day 7 of luteal phase and 24 h later, these monkeys were administered with 20 IU/ kg BW of rhLH intravenously. Blood samples were collected at different time points before and after CET+LH treatments for determining P4 levels. Since LH replacement during CET-induced luteolysis could restore luteal function, an experiment was carried out to examine earliest changes in expression of genes associated with LH-mediated rescue of CL function. Groups of monkeys (n=3/ group) were treated with VEH, CET (150 μg/kg BW), CET+PBS/ rhLH (20 IU/kg BW) or CET+rhLH 8 h. Blood samples were collected immediately before VEH/CET injections, 12 and 24 h post injections and 1 and 8 h post PBS or LH injections. At 1 and 8 h post PBS or LH injections, CL was retrieved from all monkeys for determining messenger RNA levels of luteal genes and StAR protein levels.
Experiments in Rats (Harlan Wistar)
Female rats aged 3 to 4 months were housed in a controlled environment (12 h Light: Dark cycle) with ad libitum access to food and water. The female rats were cohabitated with breeder male rats. The vaginal smears were screened daily for the presence of sperm and the day of presence of sperm was designated as day 1 of pregnancy. A number of earlier studies have reported loss of pregnancy in response to GnRH R antagonist treatment administered on different days of pregnancy. However, large variations in response to the GnRH R antagonist treatment have been observed [21]. Also, several studies have determined the requirement of LH for CL function during days 7-10 of pregnancy in rats [22].
The present studies were carried out in rats during days 8-10 of pregnancy to establish a consistent response to GnRH R antagonist. A range of doses of CET was examined and the details of experiments and results are provided in (Figure 1).
Figure 1: Effect of different doses of CET on pregnancy in rats#.
#: As denoted on the left hand side of the Table, rats were treated with a number of injections (denoted by * at 7 am and 7 pm) of CET comprising of, 150, 300 or 600 μg/kg BW. The number of rats which lost pregnancy out of the total number of rats used for each dose are shown in the circle.
Based on the results of the dose determination studies, a CET dose of 150 μg/kg BW twice daily administrations was selected for treatment during days 8-10 of pregnancy to induce luteolysis and loss of pregnancy in rats.
A group of day 8 pregnant rats was administered either VEH or CET (150 μg/kg BW, 6 injections) subcutaneous twice daily for three days and CL (n=5 rats/VEH or CET group) were collected 72 h post treatments.
In another experiment, a group of pregnant rats at the end of 48 h of initiation of CET treatment (4 injections) was administered either 100 μl of PBS (VEH) or highly purified hLH preparation (1μg/100μl) i.p., and CL (n=3 rats/PBS or LH group) from both the groups were collected at 72 h. Blood samples were collected at different time points to examine P4 levels.
RNA Isolation
Total RNA was isolated from CL tissue of different experiments using TRI reagent according to the reported procedure previously [23]. The quality and quantity of RNA was determined as reported previously [20].
Semi Quantitative RT-PCR Expression Analysis
Semi quantitative RT PCR analysis was carried out as described previously [23]. The list of primers employed along with amplicon size and annealing temperature are provided in Table 1.
| Sl. No | Gene name | Oligonucleotide sequence (5’to 3’) | Annealing temp (OC) | Product size (bp) |
|---|---|---|---|---|
| 1 | StAR | F: AAGGGGCTGAGGCAACA R: CTTCCAGCCGAGAACCGAG |
60 | 133 |
| 2 | 3bHSD | F: TGGGGAAGGAGGCCCATTCC R:CCCAGGCCACGTTGCCAAC |
67 | 122 |
| 3 | P450scc | F: CGAGGACATCAAGGCCAACGT R: CCTCTGCCCGCAGCATATCC |
64 | 128 |
| 4 | Aromatase | F: CAATACCAGGTCCTGGCTAC R: CCTCTCCAGAGATCCAGAC |
60 | 141 |
| 5 | L19 | F: GCCAACTCCCGTCAGCAGA R: TGGCTGTACCCTTCCGCTT |
60 | 154 |
| 6 | LDLR | F: CTGTGACTCAGACCGGGAC R: CGAGCCATCTTCGCAGTC |
60 | 151 |
| 7 | Inhibin-a | F: GAAGGGTAGAAGAGGGTGGG R: GCAGCACCATAGCTCACCT |
63 | 130 |
| 8 | Inhibin-bA | F: CATCACGTTTGCCGAGTCAGGAA R: GAGGCGGATGGTGACTTTGGTC |
70 | 157 |
Table 1: Primers used for Semi quantitative (RT-PCR) analyses.
Quantitative Real time PCR (qPCR) Expression Analysis
The list of primers employed in the qPCR analysis is provided in Table 2. The qPCR analysis was performed as described previously from the laboratory [24]. PCR for each sample was set up in duplicates and the average Ct was used in the ΔΔCt equation.
| Sl. No | Gene name | Oligonucleotide sequence (5’to 3’) | Annealing temp (OC) | Product size (bp) |
|---|---|---|---|---|
| 1 | hmgcs1 | F: ACGATACGCTTTGGTAGTTG R: AAGCCCTCGGTCAAAAAT |
60 | 131 |
| 2 | hmgcr | F: GGGTCAAGATGATCATGTCT R: ATTCTCTTGGACACATCTTCAG |
50 | 179 |
| 3 | ldlr | F: GAGTCCCCTGAGACATGCAT R: GGGAGCAGTCTAGTTCATCCG |
61 | 145 |
| 4 | star | F: GGCCCCGAGACTTCGTAA R: TGGCAGCCACCCCTTGA |
62 | 218 |
| 5 | p-450scc | F: ACCCAACTCGTTGGTTGGA R: CACGTTGATGAGGAAGATGGT |
62 | 131 |
| 6 | 20α-hsd | F: AGAGATAGGTCAGGCCATTGT R: ATCCCCTGGCTTCAGAGATAC |
68 | 202 |
| 7 | rpl 19 | F: CGTCCTCCGCTGTGGTAAA R:AGTACCCTTCCTCTTCCCTATGC |
62 | 214 |
Table 2: Primers used for Quantitative Real Time (qPCR) analyses.
Immunoblot Analysis
Immunoblot analysis of the total protein lysates from CL tissues was carried out as per the procedures reported previously [20].
Hormone Assays
Serum P4 and E2 concentrations were determined by specific RIA as reported previously [23]. The sensitivity of P4 and E2 were 0.1 ng/ml and 39 pg/ml, respectively, and the inter- and intra- assay variations were < 10%.
Statistical Analysis
Data were expressed as mean ± SEM. Messenger RNA and protein expression of different genes were analyzed by one-way ANOVA, followed by the Newman-Keuls comparison test ( PRISM Graph pad, version 2: Graph pad software, USA). The p value <0.05 was considered statistically significant.
Results
Example I: Effect of CET treatment on circulating P4 levels and the length of menstrual cycle
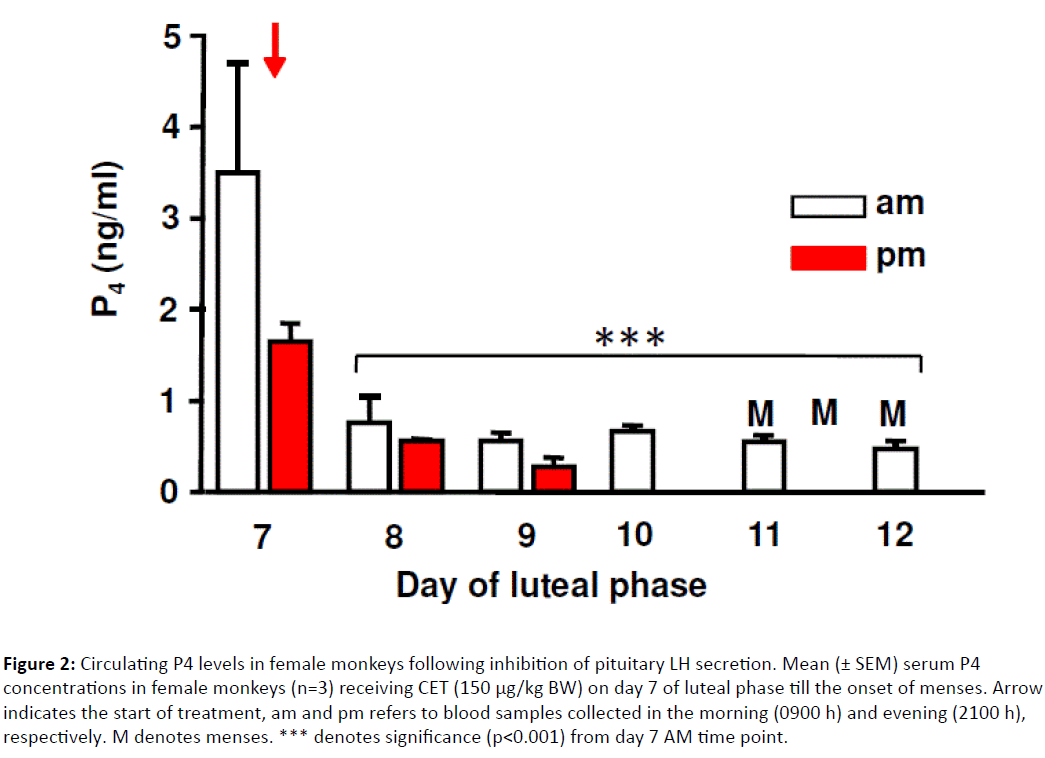
As shown in (Figure 2) , serum P4 levels prior to CET injection were 3.6 ± 1.1 ng/ml, decreased to 1.6 ± 0.1 ng/ml at 12 h, 0.8 ± 0.2 ng/ml at 24 h and remained <0.5 ng/ml thereafter. All three monkeys exhibited menstruation 96 to 120 h after CET injection corresponding to 22-23 days of menstrual cycle length during the experimental period in contrast to the menstrual cycle length of 27-29 days in monkeys not under experimentation.
Figure 2: Circulating P4 levels in female monkeys following inhibition of pituitary LH secretion. Mean (± SEM) serum P4 concentrations in female monkeys (n=3) receiving CET (150 μg/kg BW) on day 7 of luteal phase till the onset of menses. Arrow indicates the start of treatment, am and pm refers to blood samples collected in the morning (0900 h) and evening (2100 h), respectively. M denotes menses. *** denotes significance (p<0.001) from day 7 AM time point.
Example II: Effect of exogenous LH treatment on luteal function in CET-treated monkeys
Circulating P4 levels prior to CET treatment were 3.80 ± 0.41 ng/ml and decreased (p<0.05) to 1.24 ± 0.10 and 1.01 ± 0.19 ng/ml at 12 and 24 h post CET treatment (Figure 2).
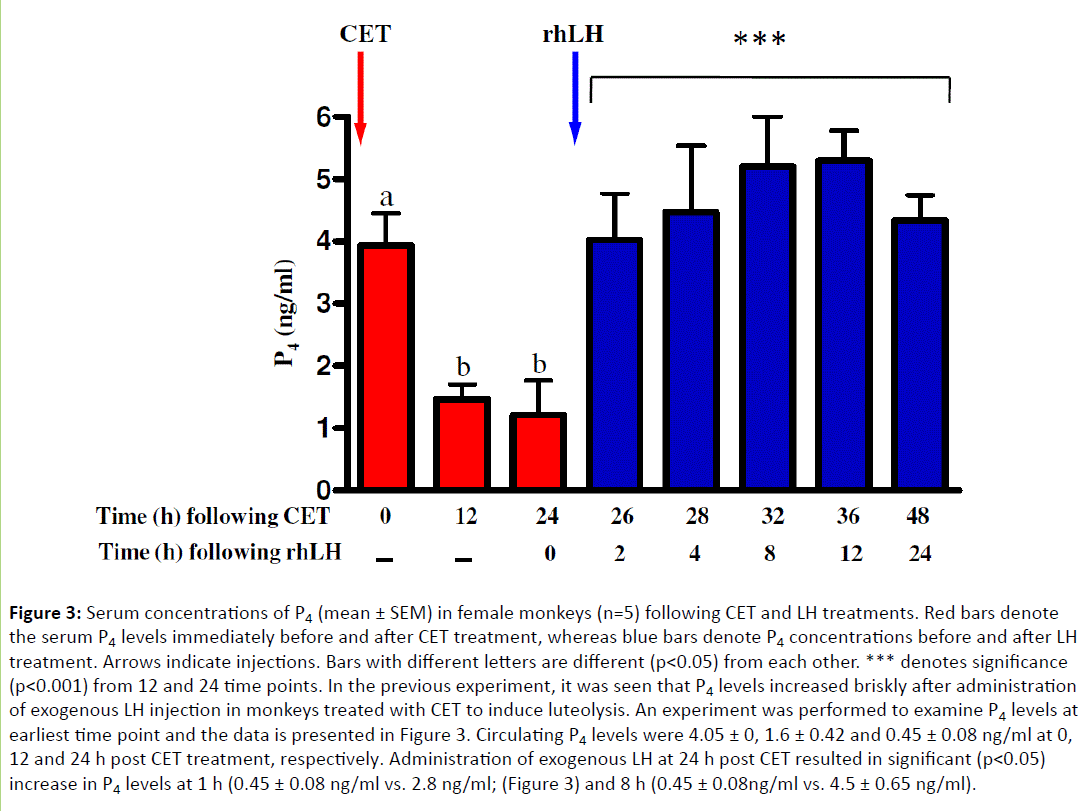
Administration of intravenous injection of rhLH increased (p<0.05) P4 to 3.8 ± 0, 4.51 ± 0, 5.2 ± 0, 5.21 ± 0.98 and 4.21 ± 0.78 ng/ml corresponding 2, 2, 36 and 48 h post CET treatment, respectively (Figure 3). Circulating P4 levels were lower at 48 h, since few monkeys exhibited menstruation 96-120 h post rhLH treatment.
Figure 3: Serum concentrations of P4 (mean ± SEM) in female monkeys (n=5) following CET and LH treatments. Red bars denote the serum P4 levels immediately before and after CET treatment, whereas blue bars denote P4 concentrations before and after LH treatment. Arrows indicate injections. Bars with different letters are different (p<0.05) from each other. *** denotes significance (p<0.001) from 12 and 24 time points. In the previous experiment, it was seen that P4 levels increased briskly after administration of exogenous LH injection in monkeys treated with CET to induce luteolysis. An experiment was performed to examine P4 levels at earliest time point and the data is presented in Figure 3. Circulating P4 levels were 4.05 ± 0, 1.6 ± 0.42 and 0.45 ± 0.08 ng/ml at 0, 12 and 24 h post CET treatment, respectively. Administration of exogenous LH at 24 h post CET resulted in significant (p<0.05) increase in P4 levels at 1 h (0.45 ± 0.08 ng/ml vs. 2.8 ng/ml; (Figure 3) and 8 h (0.45 ± 0.08ng/ml vs. 4.5 ± 0.65 ng/ml).
Example in monkeys: Effects of LH treatment on expression of luteal genes associated with steroidogenesis
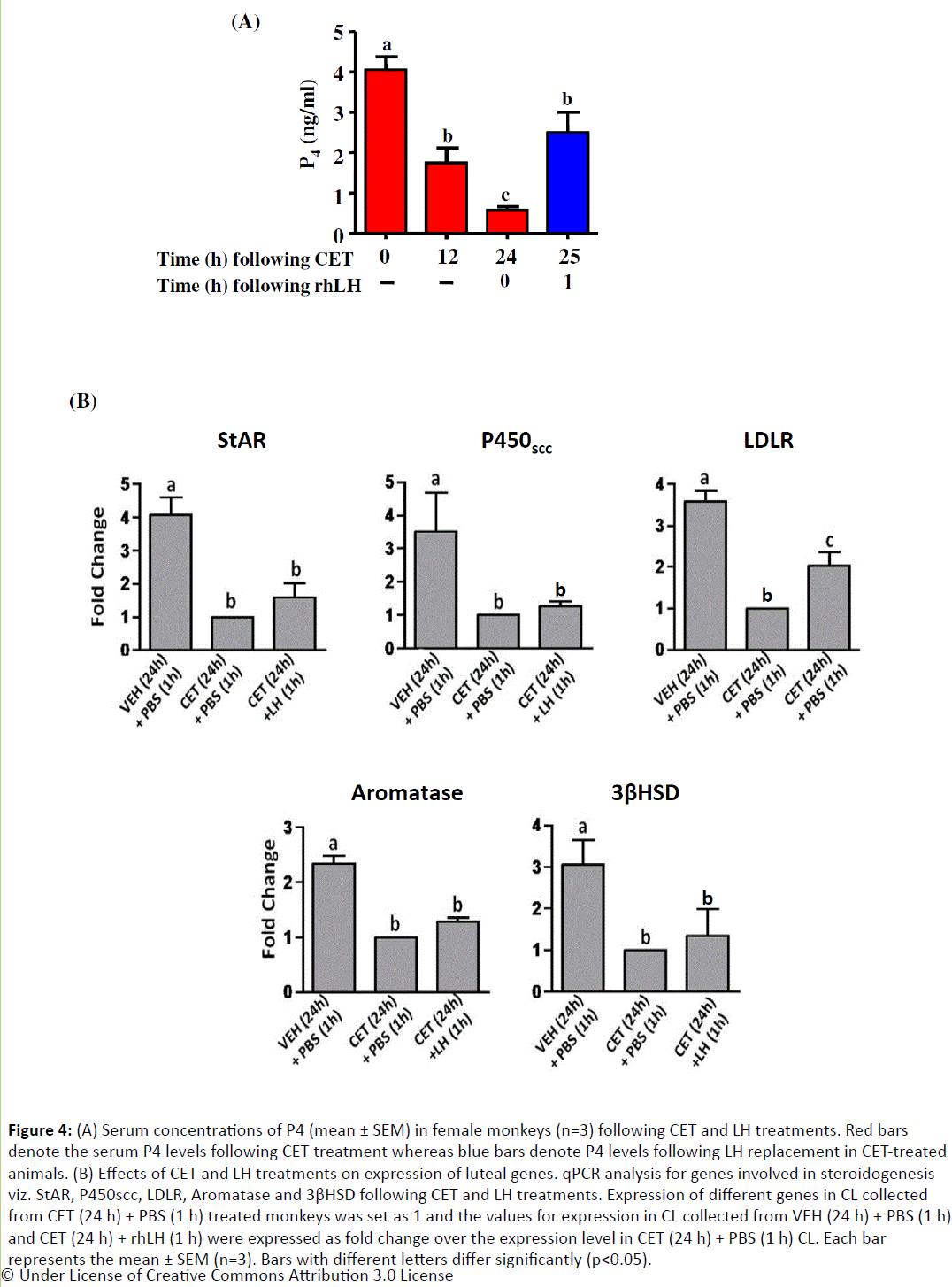
Since injection of rhLH stimulated acute increase in P4 levels in CET treated monkeys, CL from VEH/CET treated monkeys receiving LH for 1 and 8 h was examined for expression changes in steroidogenic genes and inhibin family genes. Expression of genes involved in steroidogenesis such as StAR, cholesterol sidechain cleavage enzyme (P450scc, CYP11A1), low density lipoprotein receptor (LDLR), aromatase (P450arom, CYP19A1) and 3beta-hydroxysteroid dehydrogenase (3βHSD) decreased significantly (p<0.05; Figure 4) at 24 h following CET treatment. However, there was no significant (pÃÆââ¬Â¹ÃâÃâ0.05) increase in the expression of these genes except LDLR at 1 h post LH treatment in CET treated monkeys (Figure 4), though a detectable increase in circulating P4 levels were observed. At 8 h post LH treatment, expression of aromatase, P450ssc, LDLR, StAR, 3βHSD, inhibin α and inhibin βA was significantly (p <0.05) higher compared to CL collected from monkeys treated with CET for 24 h (Figure 5).
Figure 4: (A) Serum concentrations of P4 (mean ± SEM) in female monkeys (n=3) following CET and LH treatments. Red bars denote the serum P4 levels following CET treatment whereas blue bars denote P4 levels following LH replacement in CET-treated animals. (B) Effects of CET and LH treatments on expression of luteal genes. qPCR analysis for genes involved in steroidogenesis viz. StAR, P450scc, LDLR, Aromatase and 3βHSD following CET and LH treatments. Expression of different genes in CL collected from CET (24 h) + PBS (1 h) treated monkeys was set as 1 and the values for expression in CL collected from VEH (24 h) + PBS (1 h) and CET (24 h) + rhLH (1 h) were expressed as fold change over the expression level in CET (24 h) + PBS (1 h) CL. Each bar represents the mean ± SEM (n=3). Bars with different letters differ significantly (p<0.05).
Figure 5: qPCR expression analysis of StAR, P450scc, LDLR, Aromatase, 3βHSD, Inhibin α, and Inhibin βA genes following LH replacement. The graphs represent the fold change over CET+PBS (values in this group was set as 1). Each bar represents mean ± SEM (n=3) of PCR reactions done in triplicates. Bars with different letters are significantly different (p<0.05).
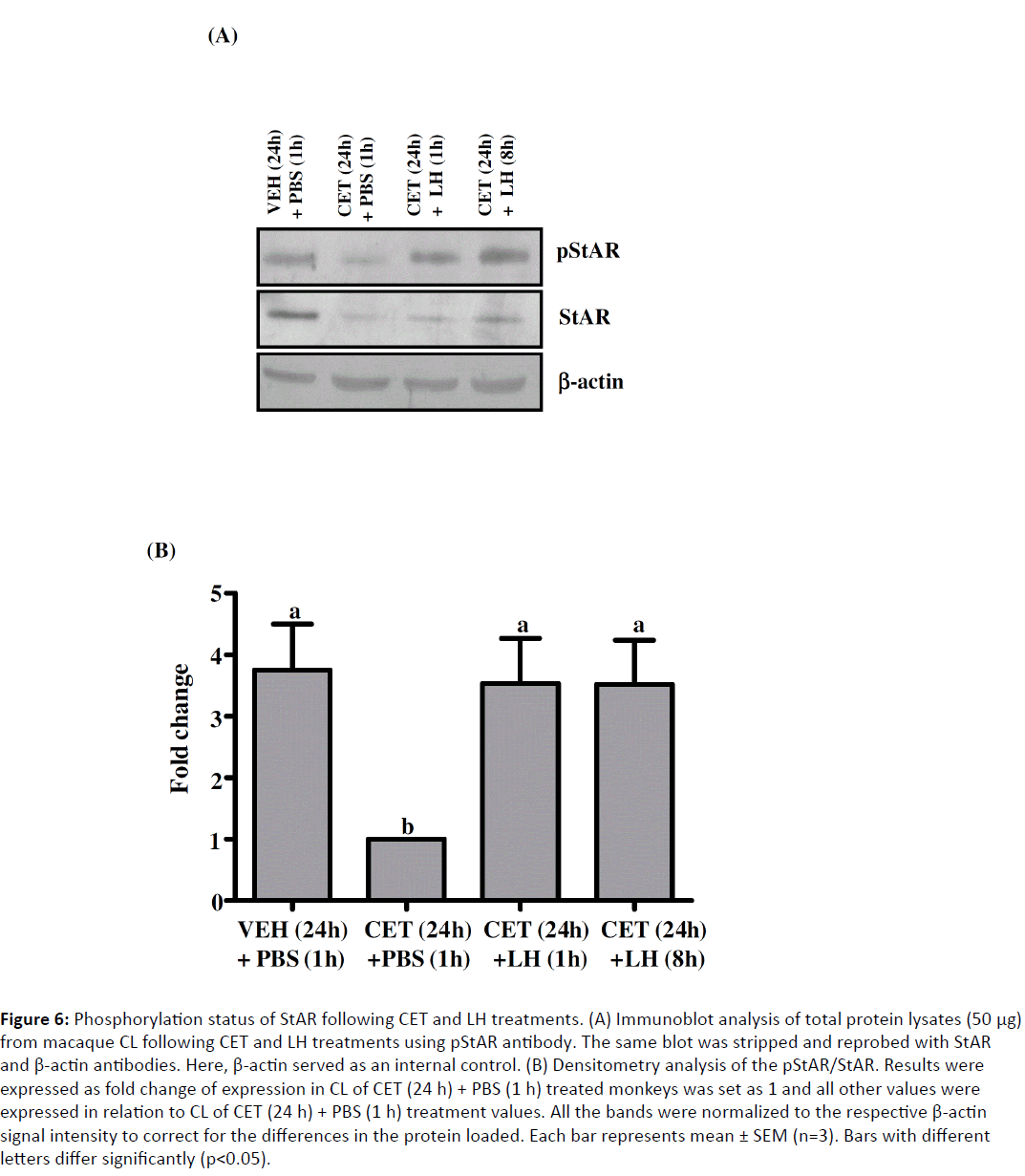
Expression of StAR, a marker of CL function and regarded as a true rate limiting step in steroidogenesis, did not increase following LH replacement, despite the increase in serum P4 levels. To study whether co or post-translational modification of StAR is responsible for this increased steroidogenesis, immunoblot analysis was performed using phospho-StAR (pStAR) antibody on CL lysates prepared from VEH (24 h) + PBS (1 h), CET (24 h) + PBS (1 h) and CET (24 h) + LH (1 and 8 h) treated monkeys. Following CET treatment, there was a dramatic decrease in StAR protein levels corroborating the mRNA expression and also the pStAR levels decreased dramatically (Figure 6). However, LH treatment did not lead to any increase in StAR protein levels which corroborates well with the mRNA expression, but it led to a significant increase in pStAR levels both at 1 and 8 h post rhLH treatment (Figure 6).
Figure 6: Phosphorylation status of StAR following CET and LH treatments. (A) Immunoblot analysis of total protein lysates (50 μg) from macaque CL following CET and LH treatments using pStAR antibody. The same blot was stripped and reprobed with StAR and β-actin antibodies. Here, β-actin served as an internal control. (B) Densitometry analysis of the pStAR/StAR. Results were expressed as fold change of expression in CL of CET (24 h) + PBS (1 h) treated monkeys was set as 1 and all other values were expressed in relation to CL of CET (24 h) + PBS (1 h) treatment values. All the bands were normalized to the respective β-actin signal intensity to correct for the differences in the protein loaded. Each bar represents mean ± SEM (n=3). Bars with different letters differ significantly (p<0.05).
Effects of CET Treatment and LH Replacement on Luteal Function in Pregnant Rats
Administration of 6 injections (at 12 h intervals) of CET treatment from day 8 of pregnancy resulted in significant (p<0.05) decrease in P4 levels from blood collected 24 h after the end of last injection of CET treatment (Figure 7I). In CL of CET treated rats, expression of hmgcr, hmcgs, ldlr and p450scc was lower (Figure 7II A-D); p<0.05) in CL of CET treated rats, expression of star (Figure 7II E) was not statistically significant and expression of 20α-hsd (Figure 7II F) was significantly higher (p<0.05; Figure 7II). In another experiment, the secretion pattern of P4 before and after CET treatment and LH treatment is represented in Figure 8A. P4 levels significantly decreased (p<0.05) post 24 h CET treatment and the levels were <10 ng/ml by 72 h (Figure 8A).
Figure 7: (I) Serum levels of P4 (mean ± SEM) in rats on day 11 of pregnancy following VEH or CET treatment. CET (150 μg/kg BW) was administered twice daily on days 8-10 of pregnancy. (II) Expression of luteal genes after CET treatment. Semi-quantitative RTPCR expression analysis (mean ± SEM) of hmgcr (A), hmgcs1 (B), ldlr (C), star (D), p450scc (E) and 20α-hsd (F). rpl-19 gene was used as internal control for equal loading of RNA. Relative expression of each gene was calculated following densitometry (*:p<0.0, **:p<0.01 and ***:p<0.001).
Figure 8: Effects of GnRH R antagonist and LH treatments on luteal steroidogenesis. (A) Serum levels of P4 (mean ± SEM) in rats on day 11 of pregnancy following VEH or CET treatment. CET (150 μg/kg BW) was administered twice daily on days 8-10 of pregnancy. (B) Serum P4 levels in rats treated with PBS or LH at 48 h after initiation of CET treatment. Arrows indicate injections of CET, PBS and LH treatments. (C) Immunoblot analysis of pStAR and total StAR expression in CL collected from rats treated with no treatment (VEH), CET+PBS and CET+LH. Densitometry data is expressed as mean ± SEM. Bars with different letters are different (p<0.05) from each other.
P4 secretion pattern before and after PBS/LH treatment in CET treated pregnant rats are shown in (Figure 8B). The P4 levels prior to CET treatment were set as 100% and the levels at other time points were expressed in relation to 100% (Figure 8B). Administration of PBS at 48 h after initiation of CET treatment had no effect on declining P4 secretion (Figure 8B), but administration of LH treatment halted the declining P4 secretion and by 72 h, P4 secretion was restored up to 60% of the concentration observed before CET treatment (Figure 8B). Immunoblot analysis of StAR (both total StAR and pStAR) expression revealed that total StAR tended to be lower in CET treated rats, but pStAR clearly showed higher expression following LH treatment (Figure 8C).
Discussion
In monkeys, a number of previous studies including ours utilized repeated administration of the GnRH R antagonists for periods up to 2 to 3 days to inhibit endogenous LH [6-10]. Although the treatment regimen was effective in inhibition of luteal function, but the possibility of CL being exposed to residual LH effects could not be ruled out between intervals of injections of antagonist treatment. Moreover, in repeated injection model systems, it will be difficult to accurately determine the time point of actual inhibition of LH secretion. In the present study, a single injection of antagonist was sufficient to inhibit LH action. Since P4 levels decreased within 3-4 h after CET treatment and by 24 h post treatment, expression of several genes associated with steroidogenesis declined to levels comparable to the CL considered regressed [11]. In women, Del Canto et al. [25] also reported use of single injection of 2 mg of CET to induce luteal regression and a number of features of induced regression has been characterized. Together with studies in women, the present results clearly demonstrate that the protocol is so simple that it could be considered for terminating the luteal phase, and has potential implications for utilization as non-surgical and pre-implantation contraception in women. It should be pointed out that other than dose determination studies reported [21], there is very limited information available on induced luteolysis in pregnant rats.
The midcycle LH surge, considered to be the physiological trigger, initiates ovulation and luteinization of preovulatory follicles via induction of specific genes in granulosa cells required for these two processes. Initially, it was believed that once the CL is formed, it functions independent of pituitary LH [26]. Also, once luteal formation is initiated, the mechanisms involved in the LH/hCG-stimulated cAMP production becomes desensitized, and therefore requirement for continued cAMP-dependent signaling is lost leading to a state of cAMP non-responsiveness in the maturing luteal cell [27-31]. It was observed that rhesus monkey luteal cells became unresponsive to exogenous gonadotropins in vitro as they proceed from mid to late luteal phase [32]. The independence of CL from circulating LH was ruled out in primates, since it was shown that neutralization of monkey LH by administering antibodies against hCG led to decrease in P4 levels and premature menses [4]. Furthermore, cessation of GnRH infusion in MBH-lesioned monkeys during early or mid-luteal phase decreased serum P4 levels followed by menses suggesting the dependence of CL on LH for its structure and function [33]. Employing GnRH antagonists, various groups have proved the obligatory requirement of LH for CL in women [34] and several species of macaques [35,36]. Although all these LH-withdrawal models were utilized for studying physiological and molecular events underlying luteolysis, these models required either continuous infusion of GnRH to MBH-lesioned monkeys or repeated injections of GnRH antagonists for at least 3 days to observe a significant decrease in serum P4 levels. In the present study, an induced luteolysis model was standardized in which administration of a single injection of CET on day 7 of the luteal phase was sufficient to initiate the process of luteolysis. Similar experiments have also been reported in humans, in which a single subcutaneous injection of 3-5 mg of CET led to a rapid and marked suppression of LH and E2 [37].
To further demonstrate the role of LH in the maintenance of primate CL function, replacement, studies were performed by various groups, in which gonadotropins were administered either simultaneously or following GnRH R antagonist treatment. It was observed that co-administration of hCG and LHRH (GnRH) R antagonist prevented the fall in P4 levels [38]. Similarly, simultaneous administration of hCG and GnRH R antagonist also could reverse the luteolysis process, whereas administration of hCG at 48-72 h post GnRH antagonist treatment could not avert the decrease in P4 and luteolysis [7]. In the studies carried out by Duffy et al. [9], only the escalating doses of rhLH along with the Antide (GnRH R antagonist) treatment could restore the P4 production. In the present study, as it was possible to induce luteolysis with a single injection of CET in monkeys and multiple injections in rats, it was further examined whether this CETinduced luteolysis process was reversible. LH replacement studies indicated that administration of a single injection of rhLH/LH was sufficient to restore P4 levels to pre-CET treatment levels. It was surprising then that CL from both the species continue to maintain sensitivity to LH during the regressing state. It should be pointed out that in both the species, the LH receptor expression became lower after CET treatment [18].
To elucidate the molecular mechanisms underlying the immediate increase in serum P4 levels, expression of genes involved in steroidogenesis were examined in both the species. It was observed that LH treatment in CET-treated monkeys did not show expression changes at 1 h, but the expression of many luteal genes had recovered from CET inhibited condition by 8 h.
In pregnant rats, expression of luteal genes decreased, excepting for StAR expression. Evaluation of the phosphorylation status of StAR protein at 1 h time point in both species showed an increase in the pStAR level suggestive of maintaining responsiveness to LH. StAR was identified as a 30 kDa phosphoprotein associated with mitochondria in gonadal and adrenal cells [39,40] and it was suggested that StAR undergoes phosphorylation in response to cAMP, generating the active form of the protein [41]. Arakane et al. [42], reported that Ser 194/1, a potential site of phosphorylation mediated by PKA, is an important residue in StAR and mutation of this residue led to a 50% decrease in its activity. The authors also proposed that, a tropic stimulus which leads to an acute increase in cAMP and subsequently activation of PKA, will lead to phosphorylation of StAR and due to its short half-life, any increase in its biological activity will have a significant but short-lived effect on steroidogenesis. It is very well known that gonadotropinmediated increase in steroid biosynthesis can be divided into two sequential steps. First, the acute effects (co or posttranslational) that occur within an hour, due to the phosphorylation of proteins critical for CL/testis function. Second, the long-term effects (transcriptional) that occur at later time points may be due to the increased transcription of genes encoding essential components of steroidogenic machinery like StAR, aromatase, 3βHSD and P450scc. It is possible that phosphorylation of StAR might have contributed to the immediate increase in P4 biosynthesis observed at 1 h following LH treatment in both the species.
To our knowledge, the present study is the first one to demonstrate the induction of luteolysis by a single injection (monkeys) or multiple injections (rats) of CET and reversal of this luteolytic event by a single injection of rhLH in both the species. These findings provide evidence for involvement of co- or posttranslational changes in the CL immediately following exposure to LH during inhibition of endogenous LH, and the data further suggests resilience of the CL structure that recovers quickly from the LH-deprived state. The new findings of LH blockade and replacement will pave the way for determining specific LH actions in the regulation of structural and functional properties of CL in both monkeys and rats.
Acknowledgements
We are grateful to Drs. RG Prakash, MK Vinuthan and GM Satheesha of Primate Research Laboratory for assistance with surgery. Priyanka Samji was supported by a fellowship from the Council of Scientific and Industrial Research, New Delhi, India. The research for this study was supported by DBT-IISc Partnership Program for Advanced Research in Biological Sciences & Bioengineering, DST-FIST and UGC-SAP, Government of India.
References
- Stocco C,Telleria C, Gibori G (2007) The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28: 117-149.
- Pate JL, Johnson-Larson CJ, Ottobre JS (2012) Life or death decisions in the corpus luteum. ReprodDomestAnim 47: 297-303.
- Stouffer RL, Bishop CV, Bogan RL, Xu F, Hennebold JD (2013) Endocrine and local control of the primate corpus luteum. ReprodBiol 13: 259-271.
- Moudgal NR, Macdonald GJ, Greep RO (1972) Role of endogenous primate LH in maintaining corpus luteum function in the monkey. J ClinEndocrinolMetab 35: 113-116.
- Hutchison JS, Zeleznik AJ (1985) The corpus luteum of the primate menstrual cycle is capable of recovering from a transient withdrawal of pituitary gonadotropin support. Endocrinology 117: 1043-1049.
- Bajusz S,Csernus VJ, Janaky T, Bokser L, Fekete M, et al. (1988) New antagonists of LHRH. II. Inhibition and potentiation of LHRH by closely related analogues. Int J Pept Protein Res 32: 425-435.
- Dubourdieu S,Charbonnel B, Massai MR, Marraoui J, Spitz I, et al. (1991) Suppression of corpus luteum function by the gonadotropin-releasing hormone antagonist Nal-Glu: effect of the dose and timing of human chorionic gonadotropin administration. FertilSteril 56: 440-445.
- Bishop CV,Bogan RL, Hennebold JD, Stouffer RL (2011) Analysis of microarray data from the macaque corpus luteum; the search for common themes in primate luteal regression. Mol Hum Reprod 17: 143-151.
- Duffy DM, Stewart DR, Stouffer RL (1999) Titrating luteinizing hormone replacement to sustain the structure and function of the corpus luteum after gonadotropin-releasing hormone antagonist treatment in rhesus monkeys. J ClinEndocrinolMetab 84: 342-349.
- Yadav VK, Medhamurthy R (2006) Dynamic changes in mitogen-activated protein kinase (MAPK) activities in the corpus luteum of the bonnet monkey (Macacaradiata) during development, induced luteolysis, and simulated early pregnancy: a role for p38 MAPK in the regulation of luteal function. Endocrinology 147: 2018-2027.
- Ravindranath N, Little-Ihrig L, Benyo DF, Zeleznik AJ (1992) Role of luteinizing hormone in the expression of cholesterol side-chain cleavage cytochrome P450 and 3 beta-hydroxysteroid dehydrogenase, delta 5-4 isomerase messenger ribonucleic acids in the primate corpus luteum. Endocrinology 131: 2065-2070.
- Collins RL, Sopelak VM, Williams RF, Hodgen GD (1986) Prevention of gonadotropin-releasing hormone antagonist induced luteal regression by concurrent exogenous pulsatile gonadotropin administration in monkeys. FertilSteril 46: 945-953.
- Fraser HM, Nestor JJ, Vickery BH (1987) Suppression of luteal function by a luteinizing hormone-releasing hormone antagonist during the early luteal phase in the stumptailed macaque monkey and the effects of subsequent administration of human chorionic gonadotropin. Endocrinology 121: 612-618.
- Stocco CO, Chedrese J, Deis RP (2001) Luteal expression of cytochrome P450 side-chain cleavage, steroidogenic acute regulatory protein, 3beta-hydroxysteroid dehydrogenase, and 20alpha-hydroxysteroid dehydrogenase genes in late pregnant rats: effect of luteinizing hormone and RU486. BiolReprod 142: 4158-4161.
- Medhamurthy R, Priyanka G, Vinuthan MK, Manjunatha AM (2007) Short-term fasting leads to inhibition of responsiveness to lh-stimulated testosterone secretion in the adult male bonnet monkey. Am J Primatol 69: 1-11.
- Suresh PS, Medhamurthy R (2009) Dynamics of circulating concentrations of gonadotropins and ovarian hormones throughout the menstrual cycle in the bonnet monkey: role of inhibin A in the regulation of follicle-stimulating hormone secretion. Am J Primatol 71: 817-824.
- Priyanka S, Medhamurthy R (2007) Characterization of cAMP/PKA/CREB signaling cascade in the bonnet monkey corpus luteum: expressions of inhibin-alpha and StAR during different functional status. Mol Hum Reprod 13: 381-390.
- Priyanka S, Jayaram P, Sridaran R, Medhamurthy R (2009) Genome-wide gene expression analysis reveals a dynamic interplay between luteotropic and luteolytic factors in the regulation of corpus luteum function in the bonnet monkey (Macacaradiata). Endocrinology 150: 1473-1484.
- Yadav VK, Muraly P, Medhamurthy R (2004) Identification of novel genes regulated by LH in the primate corpus luteum: insight into their regulation during the late luteal phase. Mol Hum Reprod 10: 629-639.
- Kunal SB, Killivalavan A, Medhamurthy R (2012)Involvement of Src family of kinases and cAMPphosphodiesterase in the luteinizing hormone/chorionic gonadotropin receptor-mediated signaling in the corpus luteum of monkey. ReprodBiolEndocrinol 29: 10-25.
- Rivier C, Rivier J, Vale W (1981) Antireproductive effects of a potent GnRH antagonist in the female rat. Endocrinology 108: 1425-1430.
- Raj HG, Moudgal NR (1970) Hormonal control of gestation in the intact rat. Endocrinology 86: 874-889.
- Jyotsna UR,Medhamurthy R (2009) Standardization and validation of an induced ovulation model system in buffalo cows: Characterization of gene expression changes in the periovulatory follicle. AnimReprodSci 113: 71-81.
- Shah KB, Tripathy S, Suganthi H, Medhamurthy R (2014) Profiling of luteal transcriptome during prostaglandin F2-alpha treatment in buffalo cows: analysis of signaling pathways associated with luteolysis. PLoS One 9: e104127.
- Del Canto F, SierraltaW, Kohen P, Munoz A, Strauss JF, et al. (2007) Features of natural and gonadotropin-releasing hormone antagonist-induced corpus luteum regression and effects of in vivo human chorionic gonadotropin. J ClinEndocrinolMetab 92: 4436-4443.
- Gemzell C (1965) Induction of ovulation with human gonadotropins. Recent ProgHorm Res 21: 179-204.
- Hunzicker-Dunn M, Birnbaumer L (1976) Adenylyl cyclase activities in ovarian tissues. III. Regulation of responsiveness to LH, FSH, and PGE1 in the prepubertal, cycling, pregnant, and pseudopregnant rat. Endocrinology 99: 198-210.
- Hickey GJ, Krasnow JS, Beattie WG, Richards JS (1990) Aromatase cytochrome P450 in rat ovarian granulosa cells before and after luteinization: adenosine 3’,5’- monophosphate-dependent and independent regulation. Cloning and sequencing of rat aromatase cDNA and 5’ genomic DNA. MolEndocrinol 4: 3–12.
- Carlone DL, Richards JS (1997) Functional interactions, phosphorylation, and levels of 3',5'-cyclic adenosine monophosphate-regulatory element binding protein and steroidogenic factor-1 mediate hormone-regulated and constitutive expression of aromatase in gonadal cells. MolEndocrinol 11: 292-304.
- Gonzalez-Robayna IJ, Alliston TN, Buse P, Firestone GL, Richards JS (1999) Functional and subcellular changes in the A-kinase-signaling pathway: relation to aromatase and Sgk expression during the transition of granulosa cells to luteal cells. MolEndocrinol 13: 1318–1337.
- Maizels ET, Mukherjee A, Sithanandam G, Peters CA, Cottom J, et al. (2001) Developmental regulation of mitogen-activated protein kinase-activated kinases-2 and -3 (MAPKAPK-2/-3) in vivo during corpus luteum formation in the rat. MolEndocrinol 15: 716-733.
- Stouffer RL, Nixon WE, Gulyas BJ, Hodgen GD (1977) Gonadotropin-sensitive progesterone production by rhesus monkey luteal cells in vitro: a function of age of the corpus luteum during the menstrual cycle. Endocrinology 100: 506-512.
- Hutchison JS, Zeleznik AJ (1984) The rhesus monkey corpus luteum is dependent on pituitary gonadotropin secretion throughout the luteal phase of the menstrual cycle. Endocrinology 115: 1780-1786.
- Hall JE, Bhatta N, Adams JM, Rivier JE, Vale WW, et al. (1991) Variable tolerance of the developing follicle and corpus luteum to gonadotropin-releasing hormone antagonist-induced gonadotropin withdrawal in the human. J ClinEndocrinolMetab 72: 993-1000.
- Webley GE, Hodges JK, Given A, Hearn JP (1991) Comparison of the luteolytic action of gonadotrophin-releasing hormone antagonist and cloprostenol, and the ability of human chorionic gonadotrophin and melatonin to override their luteolytic effects in the marmoset monkey. J Endocrinol 128: 121-129.
- Zeleznik AJ1 (1998) In vivo responses of the primate corpus luteum to luteinizing hormone and chorionic gonadotropin. ProcNatlAcadSci USA 95: 11002-11007.
- Erb K, Klipping C, Duijkers I, Pechstein B, Schueler A, et al. (2001) Pharmacodynamic effects and plasma pharmacokinetics of single doses of cetrorelix acetate in healthy premenopausal women. FertilSteril 75: 316-323.
- Krueger RJ, Orme-Johnson NR (1983) Acute adrenocorticotropic hormone stimulation of adrenal corticosteroidogenesis. Discovery of a rapidly induced protein. J BiolChem 258: 10159-10167.
- Stocco DM,Sodeman TC (1991) The 30-kDa mitochondrial proteins induced by hormone stimulation in MA-10 mouse Leydigtumor cells are processed from larger precursors. J BiolChem 266: 19731-19738.
- Lin D, Sugawara T, Strauss JF, Clark BJ, Stocco DM, et al. (1995) Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267: 1828-1831.
- Clark BJ, Ranganathan V, Combs R (2000) Post-translational regulation of steroidogenic acute regulatory protein by cAMP-dependent protein kinase A. Endocr Res 26: 681-689.
- Arakane F, King SR, Du Y, Kallen CB, Walsh LP, et al. (1997) Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J BiolChem 272: 32656-32662.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences