In Utero Exposure of Biochanin-A Alters Female Reproduction in Rat.
M.G.S. Soujanya, K. Prathap Reddy, V. Sridevi, P. Ramachandra Reddy and P.Sreenivasula Reddy
M.G.S. Soujanya1, K. Prathap Reddy2, V. Sridevi1, P. Ramachandra Reddy1* and P. Sreenivasula Reddy2
1Department of Biochemistry, Yogi Vemana University, Vemanapuram, Kadapa, A.P., India
2Department of Zoology, Sri Venkateswara University, Tirupati, A.P., India
- *Corresponding Author:
- Reddy PR
Department of Biochemistry
Yogi Vemana University
Vemanapuram, Kadapa – 516 003
A.P., India
Tel: +91-08562-225425
Fax: +91-08562-225419
E-mail: reddyprbiotech@gmail.com
Received date: April 11, 2016; Accepted date: April 22, 2016; Published date: April 24, 2016
Citation: Soujanya MGS, Reddy KP, Sridevi V, Reddy PR, Reddy PS (2016) In Utero Exposure of Biochanin-A Alters Female Reproduction in Rat. Journal of Clinical and Molecular Endocrinology 1:8 doi: 10.21767/2572-5432.100009
Copyright: © 2016 Soujanya MGS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Biochanin-A (BCA) is an isoflavone chemically called as 4-methyl genistein. It is plant derived non-steroidal compound, possesses estrogen like biological Activity The present study focussed on the changes of Reproduction in female rats exposed to BCA during the perinatal period.Pregnant rats were intra peritoneally exposed to 25 mg, 50 mg and 100 mg/kg body weight (wt.) on gestation days 12th, 14th, 16th and 18th. Rats were allowed to deliver pups and the BCA exposure was continued lactationally till weaning. Female pups were separated and fed with normal pellet for 80 days and then estrus cycle length was measured. Significanƚ increase in the duration of the estrus cycle was measured in rats exposed to BCA during the perinatal period. To assess the Reproductive performance of BCA exposed female progeny, mated with 90days old normal males. Observed increase in the Conception time and decrease in the number of implantations and also increase in pre and post implantations loss. To confirm the BC-A induced Reproductive Abnormalties in females computatinally docking studies was performed with aromatase and β estrogen receptor (β-ER) and in comparison with its substrates androstenedione and estradiol respectively along with BC-A and found strong competitive inhibition by BC-A with the substrates of aromatase and β-ER. It is very clear from the result that pre and post natal exposure of BCA suppresses the female reproduction in rats..
Keywords
Phytoestrogen; Biochanin-A; Estrus cycle; Conception time; Implantation-loss; Aromatase; β estrogen receptor
Introduction
Phytoestrogens are non-steroidal compounds which mimic like estrogen in animal systems [1] and are structurally similar to 17 β-estradiol (E2) which is a mammalian estrogen [2,3]. Structurally phytoestrogens are classified in to four main categories: iso-flavonoids, flavonoids, stilbenes and lignans. Isoflavones are found mainly in soy and red clover, in the form of glycosides (genistein and daidzein). Besides glycosides, isoflavones are also found in three different forms, aglycones (unconjugated), acetyl glycosides and malonylglycosides [4]. Once ingested, genistin and daidzein get rapidly metabolized in the gut by β-glucosidases to their aglycone form, i.e., genistein and daidzein [5,6]. Genistein and daidzein are also formed by bacterial de-methylation of biochanin A and formononetin, respectively [7]. Isoflavonoids are comparatively similar to 17β-estradiol, and are having binding affinity for both ER-α and ER-β [8]. Similarly most of phytoestrogens bind to ERβ [9].
These phytoestrogens act as both agonists and antagonists by mimicking the mammalian estrogen (E2) [10]. Mostly phytoestrogens are shown to bind with two types of estrogen receptors, estrogen receptor-α (ER-α), estrogen receptor –β (ER-β) from rats [11] and humans [12]. Phytoestrogens are having stable structure and low molecular weight, due to this they can pass through the cell membrane and can bind to estrogen receptors thereby induce the expression of estrogenresponsive gene products and alter the steroid hormone metabolism [13,14].
Biochanin-A and formononetin are the 4-O-methyl derivatives of genistein and daidzein respectively, and the more dominant isoflavones found in alfalfa [15], red clover, chick peas [16] and in other legumes. Isoflavones exert beneficiary effects in prevention of cancer, cardiovascular diseases and osteoporosis. It is well reported that isoflavones can adversely affect development, male and female reproduction [17,18] and it was proved in rats [19,20] and male mice. The phytoestrogen genistein exposure causes the abnormalities in the estrus cycle length, due to the abnormal function of hypothalamic-pituitary-ovarian axis function [21]. BCA prevent chemical induced tumour carcinogenesis and tumour growth after implantation in animals [22-24]. Besides anti-cancers properties isoflavone also reduce the loss of bone mineral density in females [25] and lower LDL cholesterol in males. But no clear reports are there on BCA induced reproductive abnormalities in rats.
Lot of information is available on phytoestrogens in the web [26] listed the databases related to phytoestrogens. The most prominent database for information on isoflavones is available with USDA, United States (2008). In vitro studies revealed that most phytoestrogens, including the isoflavones activate ERdependent gene transcription by binding both α-ER and β-ER, and both, α-ER and β-ER have a higher relative binding affinity with isoflavones [27-29].
To identify the female reproductive abnormalities caused by any agent the most common molecules to be studied are estrogen receptors (ERs) and the female specific enzyme aromatase and their interactions with endocrine disruptors (EDs). Though there are two ER subtypes α-ER and β-ER, using for in vitro studies, the dominant subtype, which responds predominantly to phytoestrogens is β-ER [30,31]. The studies performed with β-ER are clearly indicating the reasons why the female reproduction is disturbing by the endocrine disruptors including phytoestrogens [30,31].
The second molecule is the enzyme aromatase converts androstenedione to estrone in ovarian tissue, mostly used for inhibiting the said conversion in postmenopausal women. In women estrogen biosynthetic pathway aromatase is a rate limiting enzyme. Interaction studies (docking) of phytoestrogens with β-ER and aromatase computationally will reveal the mechanism of action of phytoestrogens in suppression of female reproduction.
The present study is focused on BC-A induced reproductive abnormalities in the female rats exposed during gestation and lactation, and to support the results docking studies is also performed computationally in between the phytoestrogens and the marker molecules β-ER and aromatase along with their respective substrate molecules.
Materials and Methods
Chemicals
The test chemical BCA was purchased from Sigma Aldrich, St Louis, MO, USA, and all other chemicals were purchased from Merck, Mumbai, India and HiMedia private Limited Laborateries, Mumbai, India.
Maintenance of experimental animals
Healthy Wistar strain rats were purchased from authorized dealer (M/S Raghavendra Enterprises, Bangalore, India). Rats were housed in polypropylene cages (18" × 10" × 8") lined with sterilized paddy husk, and provided filtered tap water and standard rat food (purchased from SaiDurga Agencies, Bangalore, India) (ad libitum). They were maintained in a wellcontrolled environment (temperature 25 ± 2°C; 12-hour light and 12-hour dark cycle, humidity 50 ± 10%). The experiments were carried out as per with the guidelines given by the Committee for the Purpose of Control and Supervision on Experiments on Animals, Government of India (CPCSEA, 2003) and approved by the Institutional Animal Ethical Committee at Sri Venkateswara University, Tirupati, India (Resolution No. 10/(i)/a/CPCSEA/IACE/SVU/PSR-MRA).
Biochanin-A exposure
A total of twenty four female rats were mated with control males and are divided in to four groups of each having six animals. The first group served as control and received 100% DMSO. The second, third and fourth groups are served as experimental groups and received 25 mg, 50 mg and 100 mg BCA (dissolved in 100% DMSO) per kg body weight respectively on 12th, 14th, 16th and 18th day of gestation. All the rats were fed with normal pellet diet and were allowed to deliver pups. The BCA exposure was continued during lactation until weaning.
The female pups of each group were separated, maintained in separate cages and fed with normal pellet diet. Then estrus cycle length was measured at the age of 80 days and fertility studies were conducted by mating normal mature (90 days old) male rats.
Length of estrus cycle
The reproductive cycle of female rats is called estrous cycle and is characterized as proestrous, estrous, metaestrous and diestrous [32,33]. The ovulation occurs from the beginning of proestrous to the end of estrous [34,35]. From the onset of sexual maturity up to the age of 12 months, the mean cycle length of estrous cycle in the normal female rat is 4 days [32,33,36].
Three successive estrous cycles were measured both in controls and experimental females by vaginal smear test [37]. The vaginal smear of all female rats was examined by the method described by [37] and reviewed by [38]. Different stages of estrous cycle were observed in all the females early in the morning by collecting vaginal fluid. In brief vaginal smear was taken by injecting few drops of normal saline (0.9% NaCl) in to the vagina, collected with a micro-tipped pasture pipette and placed on a clean plain glass slide. The cells in the smear were observed under a phase contrast microscope (Olympus, Model no: BX41TF, USA) so as to identify the different phases of estrous cycle.
Fertility test
After the measurement of three successive estrous cycles, control and experimental females were co-habited with normal males in a ratio of 1:1. The presence of vaginal plugs or spermatozoa in the vaginal orifice was considered as successive mating and also as gestation day one (GD1). Food consumption, weight gain and clinical signs of toxicity were measured daily in pregnant rats throughout the experimentation. Six rats from each group were sacrificed by cervical dislocation on day six of gestation (GD6).
The peritoneal cavity and uterus were opened and the number of implantation sites and resorption sites were recorded [39]. The ovaries were isolated and the number of corpora lutea was determined. The remaining animals were sacrificed on day 18 of gestation (GD18) and the numbers of live and dead foetuses in the uterus were determined and no attempt was made to further characterize the dead or resorbed foetuses. All live foetuses removed from the uterus were sexed, weighed and inspected for external malformations.
Data were analyzed to determine the pre-implantation loss (difference between the number of corpora lutea and the number of implantation sites, expressed as per number of corpora lutea), and post -implantation loss (difference between the number of implantation sites and the number of live fetuses, expressed as per number of implantation sites) by following formula:
In silico analysis
In order to find out the role of BC-A in female reproduction, in silico analysis was carried out to the enzyme human aromatase and β-Estrogen receptor.
Study of aromatase
A key enzyme aromatase is involved in conversion of androstenedione in to estrone in ovarian steroidogenic pathway in females. We selected aromatase for biding studies with BC-A.
Energy minimization for aromatase
The crystal structure of aromatase enzyme (PDBID: 3POL) of Human was downloaded from Protein Data Bank and water molecules were removed. The 3POLwas energy minimized using MOE 2008 version. All hydrogen atoms were included before running energy minimization program. For energy minimization MMFF94X force field was used. 0.05 Gradient was set with a cutoff of 8-10.
Docking of aromatase with biochanin-A
Molecular docking studies were performed with BC-A and Andostenedione against energy minimized estrogen receptor (PDB ID: 1ZQ5) using Dock algorithm present MOE 2008 software. The molecular interactions between protein and ligands were interpreted using PyMOL.
Study of β-Estrogen receptor
In addition to aromatase, human β-Estrogen receptor was also used for in silico studies.
Energy minimization for β-estrogen receptor
The crystal structure of β-Estrogen receptor protein (PDBID: 3POL) of Human was downloaded from Protein Data Bank and water molecules were removed. The 3POLwas energy minimized using MOE 2008 version. All hydrogen atoms were included before running energy minimization program. For energy minimization MMFF94X force field was used, 0.05 Gradient was set with a cutoff of 8-10.
Docking studies of β-estrogen receptor with biochanin-A
Molecular docking studies were performed with BC-A and estradiol against energy minimized estrogen receptor (PDB ID: 1ZQ5) using Dock algorithm present MOE 2008 software. The molecular interactions between protein and ligands were interpreted using PyMOL.
Statistical analysis
The data were analysed statistically by using the statistical programme Instat and data was processed with two- way ANOVA followed by student’s t-test. The p<0.05 considered as significant.
Results
Throughout the experiment no mortality was observed both in control and treated groups. Increased aggressive behavior during development of females (from pups to adult stage) exposed prenatally to BC-A was observed with increased concentrations of BC-A (25 mg, 50 mg and 100 mg/kg body weight) when compared to control females. No significant net body weight gain and food consumption was observed in BC-A exposed females than the control rats (data not shown). In control and BC-A exposed females the length of three successive estrous cycles was measured. There is a significant increase in the estrous cycle length in in utero BC-A exposed females when compared to the controls (Table 1).
| Group | Estrous cycle length |
|---|---|
| Control | 5.08a ± 0.69 |
| 25 mg BCA /kg BW | 5.57b ± 0.69 (9.64) |
| 50 mg BCA /kg BW | 6.32c ± 1.01 (24.41) |
| 100 mg BCA /kg BW | 7.32d ± 0.95 (44.09) |
Table 1: Change in the length of estrous cycle in females exposed to BC-A in utero. Values are mean ± S.D of six individuals. Values in the parentheses are percent changes from control. Mean values with the same superscript do not differ significantly from each other. a - not significant; b, c anddare significant at p<0.05, p<0.001 and p<0.0001 respectively.
Increase in the length of estrous cycle is in a dose dependent manner in the experimental rats (25 mg/kg body wt. 9.64%; 50 mg/kg body wt. 24.41% and 100 mg/kg body wt. 44.09%) (Table 1). There were no significant changes observed in the duration of the first three phases of estrous cycle length i.e., proestrous, estrous and metaestrous phases in BC-A exposed females with controls, but the change is significant (p < 0.001) in diestrous phase (Figure 1).
In the three successive estrous cycles, the mean length of the diestrous phase observed for control females is 2.08 ± 0.16, and for experimental females is 2.41 ± 0.16, 2.83 ± 0.43 and 3.41 ± 0.31, respectively to 25 mg, 50 mg and 100 mg BCA/ kg body weight exposure. The mating ratio of control males with control and experimental females in the present experiment is 1:1 (Table 2).
| Parameters | Control | 25 mg/kgBW | 50 mg/kgBW | 100mg/kgBW |
|---|---|---|---|---|
| Mating index (%) | 100 (6/6) | 100 (6/6) | 100 (6/6) | 100 (6/6) |
| Conception time (days) | 1.33a ± 0.51 | 2.17b ± 0.41 (63.16) |
3.83c ± 0.98 (187.97) |
6.17d ± 0.75 (363.91) |
| Fertility index (%) | 100 (6/6) | 100 (6/6) | 83.33 (5/6) | 66.66 (4/6) |
| No. of corpora lutea/rat | 13.33a ± 0.81 | 13.17a ± 0.81 (-1.20) |
12.8a ± 0.84 (-3.97) |
12.5a ± 0.58 (-6.22) |
| No. of implantations/rat# | 11.67a ± 0.52 | 10.83ab ± 0.98 (-7.18) |
9.4b ± 0.55 (-19.45) |
7.25c ± 0.50 (-37.87) |
| Pre- implantation loss (%)# | 12.45 | 17.78 | 26.56 | 42.0 |
| No. of live fetuses/rat# | 11.33a ± 0.58 | 10.33a ± 0.58 (-8.82) |
8.67b ± 0.58 (-23.47) |
6.5c ± 0.71 (-42.63) |
| No. of resorptions/rat# | 0a | 0.67b ± 0.58 | 1.33b ± 0.58 | 3.33c ± 0.58 |
| Resorptions index (%) | 0 | 6.19 | 14.15 | 45.93 |
| Post- implantation loss (%)# | 2.91 | 4.62 | 7.76 | 10.34 |
Table 2: Effect of in utero exposure of Biochanin-A on reproductive performance of female rats.Values are mean ± S.D of six individuals; # n = 3. Values in the parentheses are percent changes from that of control. Mean values with the same superscript do not differ significantly from each other. a - not significant; b, c and d are significant at p < 0.05, p < 0.001 and p < 0.0001 respectively.
Significant increase in the conception time was observed in all the experimental groups when compared to the control group. The percent change in the conception time is also significant among the experimental groups i.e., 25 mg BC-A/kg body wt.received (63.16%) to 50 mg BC-A/kg body wt. received (187.97%) and to 100 mg BC-A/kg body wt. received (363.91%) (Table 2).
The measured fertility index revealed that the decrease in percent change is significant in 50 mg and 100 mg BC-A/kg body wt. exposed females (16.67% and 33.34% respectively) when compared to control and 25 mg BC-A/kg body wt. received females (Table 2).
From all the groups the ovaries were dissected out, weighed wet and corpora lutea was calculated and found no significance from control to experimental groups (Table 2).
The number of implantations was decreased significantly from controls (11.67 ± 0.52) to BC-A exposed experimental rats (Table 2).
Significance in decrease of implantation sites was also observed among the experimental groups (25 mg BC-A/kg body wt. received > 50 mg BC-A/kg body wt. received > 100 mg BC-A/kg body wt. received; 10.83 ± 0.98 > 9.4 ± 0.55 > 7.25 ± 0.50 respectively).
The percentage of pre-implantation loss was observed to be the significant (p < 0.001) at the dosage of 50 mg and 100 mg BC-A/kg body wt. when compared to control females and is almost 42.0% loss was recorded in 100 mg BC-A/kg body wt. exposed females.
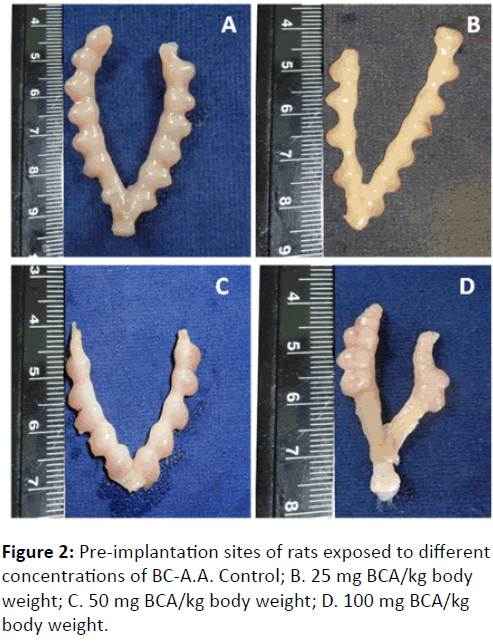
Figure 2 clearly showing the pre-implantation loss in 100 mg (D), 50 mg (C) and 25 mg (B) BC-A/kg body wt. exposed rats in contrast with control (A) rats.
The number of live fetuses was also counted in this study and significant (p < 0.001) decrease was observed in 50 mg (8.67 ± 0.58) and 100 mg (6.5 ± 0.71) BC-A/kg body wt. exposed females when compared to control and 25 mg BCA/ kg body wt. exposed rats.
The decrease in the percent change of number of live fetuses was also significant among 50 mg (-23.47%) and 100 mg (-42.63%) BC-A/kg body wt. exposed females.
The significant increase (p < 0.001) in the number of resorption sites was observed in BC-A exposed females when compared to controls (Table 2).
Significant in increase in the number of resorption sites was also observed among the experimental groups (25 mg BC-A/kg body wt. received > 50 mg BC-A/kg body wt. received > 100 mg BC-A/kg body wt. received; 0.67 ± 0.58 > 1.33 ± 0.58 >3.33 ± 0.58 respectively).
The increase in the percentage of resorption index is significant among the 50 mg and 100 mg BC-A/kg body wt. (14.15% and 45.93% respectively) when compared to control and 25 mg BC-A/kg body wt. (6.19%) exposed females.
Similarly the percentage of post implantation loss was significant in 100 mg BC-A/kg body wt. exposed rats, followed by 50 mg and 25 mg BC-A/kg body wt. exposed rats when compared to control females (10.34%, 7.76% and 4.62% respectively) (Table 2) and is shown in the (Figure 3).
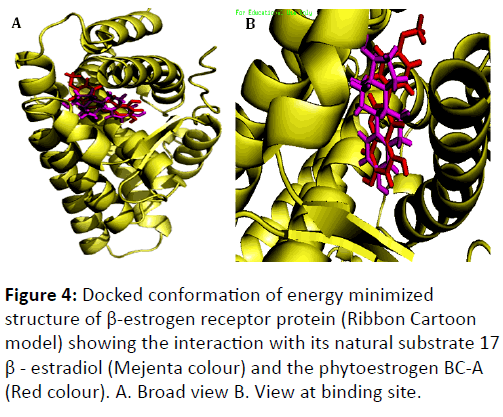
Docking studies on energy minimized structure of human aromatase with BC-A and androstenedione was performed computationally and found that BC-A has greater binding affinity (-15.3416 kcal/mol) than androstenedione (-10.0146 kcal/mol) (Table 3 and Figure 4 interacts with aromatase enzyme competitively. BC-A and androstenedione are in contact with aromatase by 14 amino acids.
| Ligands | Aromatase | β-Estrogen receptor protein |
|---|---|---|
| Estradiol (ER Substrate) |
--- | -11.8748 |
| Androstenedione (Aromatase substrate) |
-10.0146 | --- |
| Biochanin-A | -15.3416 | -12.9531 |
Table 3: Binding energies of aromatase and ß-Estrogen receptor protein with Biochanin-A and its substrates Androstenedione and Estradiol.
The interacting amino acids of aromatase with BC-A are IsoLeu -133, Phe -134, Arg -115, Leu -152, Ala -306, Val -370, Leu -372, Val -373, Leu -477, Ala -438, Cys -437, Gly -439, Thr -310, Cys -437. The amino acids of aromatase which interact with androstenedione are Arg -115, Phe -134, IsoLeu -133, Trp -224, Phe -221, Leu -372, Val -373, Met -374, IsoLeu -305, Ala -306, Asp -309,Thr -310, Ser -478, Leu -477. It seems that BCA may competitively inhibit the binding of androstenedione with aromatase.
Both BCA and estradiol interacts with estrogen receptor. Total 19 amino acids are involved in the interaction of estradiol with β-estrogen receptor and are; Thr -299, Leu -298, Met -295, Leu -301, Ala -302, Glu -305, Met -336, Leu -339, Met -340, Leu -343, Arg -346, IsoLeu -373, IsoLeu -376, Phe -377, Leu-380, His -475, Leu -476, Phe -356, Gly -472. Similar to estradiol, BC-A also have interaction with 19 amino acids of β- estrogen receptor such as; Met -295, Thr -299, Leu -298, Leu -301, Ala -302 , Glu -305, Phe -356, Leu -339, Leu -343, Met -336, Arg -346, Met -340, IsoLeu -373, IsoLeu -376, Gly -472, His -475, Leu -476, Met -479, Val -484. It seems for the estrogen receptor-β the BC-A acts as competitive inhibitor for its natural substrate estradiol.
Similarly docking studies were also performed in silico in between energy minimized structure of human β-estrogen receptor with BC-A and estradiol and found that BC-A binds with greater binding affinity (-12.9531 kcal/mol) than that of estradiol (-11.8748 kcal/mol) (Table 3 and Figure 5).
Discussion
Many environmental contaminants (Natural and manmade chemicals called xenoestrogens) are directly affecting both wild life and human health in recent years. Many of them are acting as endocrine disrupting chemicals (EDCs) and capable of affecting almost all hormonally regulated functions especially reproduction [40]. Reports are also well established on phyto and xenoestrogens caused infertility in humans and rats (implantation, gestation, and fetal growth) [41-44]. The research on phytoestrogens demonstrates that their effects on the female genital tract depend on the age at which exposure occurred and its duration. Neonatal exposure results in severe alterations in the reproductive physiology of females (Burton and Wells). The effect of phytoestrogens (isoflavones) also depends on the level of endogenous estradiol, since isoflavones and estradiol are comparatively similar and competing for their binding on ERs. If high concentrations of endogenous estrogens level is occurred (e.g. women in the follicular phase of the menstrual cycle), isoflavones may block full estrogen activity by occupying a site of the ERs. If low concentrations of endogenous estrogens occurred (men, women in menopause, after ovariectomy, etc.), the estrogen activity of isoflavones may become clear [30,45,46].
The role of phytoestrogens in causing infertility was recognized by signs of estrogenism, including mammary development, swelling of the vulva, discharge of cervical mucus, and enlargement of the uterus [47]. The present study was focused to elucidate the prenatal effect of BC-A a phytoestrogen on reproductive health of female rats. During the experimentation the female rats were exposed to BC-A (25 mg, 50 mg and 100 mg/kg body wt.) in utero, and observed aggressive behavior in a dose dependent manner. The aggressiveness is not only restricted to the female parent it was also recorded in the female young ones during development. The aggressive behavior in female rats was observed in many studies where the animals are exposed to xenoestrogens and phytoestrogens [45]. Studies conducted on animal and human beings stating that the estrogenic activity of phytoestrogens are shown to be on the adult hypothalamicpituitary- gonadal axis which finally leads to hormonal imbalance followed by infertility [21]. However aggressive behavior of injected and exposed females may be due to imbalanced hormonal regulation caused by the external agent(s), in this study may be BC-A.
The estrous cycle length measured in the present study was increased with increased concentrations of BC-A in rats. In the estrous cycle especially the di-estrous phase length was increased significantly in a dose dependent manner. Altered timing of pubertal onset and estrouscyclicity following perinatal phytoestrogen exposure has been reported in rodents [21,48-50]. Hooper et al. [51] proved that isoflavone intake increases the menstrual cycle length bysuppressing the LH and FSH levels in pre-menopausal women. Similarly the BCA exposure may increase the estrous cycle length by decreasing the hormones in rats, which may leads to prolonged di-estrus phase. Jefferson et al. [21] studied the neonatal exposure of phytoestrogen genistein on female reproduction in mice and observed the abnormal estrous cycles and ovulation. Altered ontogeny of hypothalamic kisspeptin signalling pathway and ovarian development in the female rat exposed to genisteinneonatally was also studied [52]. The timing of pubertal onset and estrouscyclicity is regulated by hypothalamic kisspeptin signalling pathways and gonad regulating hormone (GnRH) and explains abnormal estrouscyclicity. Henry and Witt [53] was studied the disruption in the estrous cycle by resroveratrol in adult rats. Perinatal exposure to genistein in female mice (10 mg/kg body wt.) or rat (0, 5, 100, 500 ppm) accelerates vaginal opening, advances pubertal onset by altering estrous cycle and also increases the length of the estrous cycle [54]. More recently in female mice and rat perinatal genistein exposure accelerates the onset of persistent estrus, causes abnormal estrous cycles, decreases fertility, delays parturition [55] and decreases the number of live pups in adulthood [21]. Similarly Murthy et al. [56] demonstrated the administration of benzene extract from flowers of H. rosasinensisintraperitoneally to adult female albino mice and found an irregular oestrous cycle in specific prolonged oestrous and metestrous with a dose dependent manner and also observed the decrease in the number of Graafian follicles, an absence of corpora lutea, and an increased number of atretic follicles and this may be the way that BC-A decreasing the diestrous phase in the exposed female rats.
In the present study, though the mating ratio of male and female (i.e., 1:1) is same in the control and BC-A exposed groups, the measured conception time was significantly increased in a dose dependent manner in BC-A exposed females than the controls. Significant change in fertility index of 50 mg and 100 mg BC-A/kg body wt. exposed females was observed and no significant change was observed in 25 mg BCA/ kg body wt. exposed females when compared to controls. Conception time was observed to be significant among the experimental groups. Similar studies were reported in rat and in mice. Pushpalatha [57] observed the significant decrease in the conception time by mating of normal males with in utero proluton depot exposed females. Significant change in the conception time and fertility index in BC-A exposed females is may be due to the decreased fertility by the phytoestrogen BCA.
Some of the phytoestrogens like genistein exposure during development period disturbs the fertility of females [21,58]. Genistein even at environmentally relevant doses neonatally had shown adverse effects on female development and reproduction in CD-1 mice [21]. No change in the weight of corpus lutea was observed in control to experimental groups indicates that the BC-A has no direct effect on corpus lutea of female rats. However BC-A has significant effect on implantation sites. BC-A exposure decreases the number of implantations in a dose dependent manner.
Neonatal genistein can also interfere with ovarian differentiation resulting in ovarian malformations indicative of impaired fecundity such as multi-oocyte follicles, and attenuated oocyte cell death [58,59]. Ovarian defects, including the absence of corpora lutea, the presence of large antral-like follicles with degenerating or no oocytes and numerous ovarian cysts have also been observed following neonatal genistein exposure in rats [48]. The obstruction of estrogen receptors by phytoestrogens, prevents the growth of follicles and hence, reduce the number of corpora lutea [60]. Though BC-A is not showing the effect on corpus lutea, it decreases significantly the implantation sites, number of live fetuses with increased post-implantation loss which ultimately increases the pre-implantation loss, number of resorptions and resorption index. This is all may be due to the BC-A caused deterioration of follicles in the exposed females.
Numerous studies have been shown DNA methylation in the germ cell ensued increase in pre-post implantation losses [61,62]. In this way isoflavones are being increasingly used as an alternative for hormonal replacement therapy in post - menopausal women, especially in cases of long-term administration [63]. In the present study loss of pre and post– implantations are significant and decrease in dose dependent manner. The number of live fetuses observed to be decreased significantly in females exposed in utero to 50 mg and 100 mg BC-A/kg body weight when compared to controls and no change was seen with females exposed to 25 mg BC-A/kg body wt. in utero. Implantation of blastocyst in the uterine endometrium is the basic feature of mammalian reproduction. Estrogen and progesterone play a main role in synchronizing the oviductal transport of pre-implantation embryo and development of a receptive uterus. The absence of estrogen in the pregnant rat at the time of implantation induces a state of dormancy of the embryo, and implantation is delayed. However if the rat is maintained on progesterone, a single injection of a very low amount of estrogen can induce implantation [64-66].
In animal models anti-fertility and abortifacient activities of phenolic and phytosterols have been confirmed [67]. The resorption index and post-implantation loss shows correlation between the number of implanted blastocysts and those that have not developed [68,69]. The reduction in the resorption index in the post-implantation losses shows the abortifacient or foetal resorption properties. Similar findings were reported by Dabhadkar D [70] inplumeriarubra pods extract and by Yakuba MT [71] in Sennaalata leaves. Increase in the resorption index shows the failure in the development of embryo. Such occurrence of fetalresorption suggests that interruption of pregnancy occurred after implantation of the fetus [72].
Basically phytoestrogens are observed to inhibit the hypothalamic-pitutary-gonad axis for secretion of leutinizinghoromone releasing hormone (LHRH) there by leutinizing hormone (LH). LH is involved in the ovulation of follicles by secreting the progesterone from corpus luteum. Exposure of phytoestrogens causes the permanent oestrous in mammalian females by inhibiting the secretion of progesterone. In general phytoestrogens are less potent ER agonists than endogenous estrogens [73]. But in the present computational docking studies the isoflavonoid BC-A has showed high binding energy with β-ER than the endogenously occurring estrogenestradiol and the interaction between the β-ER with BC-A and estradiol is with 14 amino acids. Similarly docking of BC-A with an aromatase, rate limiting enzyme in estrogen biological synthetic pathway and its substrate androstenedione and revealed that the binding energies of aromatase is higher with BC-A than its natural substrate. There are 19 amino acids of aromatase is interacting with both BC-A and androstenedione. Though the number of interacting amino acids of β-ER and aromatase with BC-A is same as with their natural substrates, there is a change in the interacting amino acids and due to change in the amino acids interacting, the binding energies with BC-A are higher than their natural substrates. Because of this may be BC-A acting as a tough competitive inhibitor for estradiol on β-ER and for androstedione on aromatase and which may responsible for endocrine disruption followed by suppression in the female reproduction.
The obtained alterations in the reproductive parameters in BC-A exposed females are well supported by the docking studies. Though 25 mg BC-A/Kg body wt. is not most effective to cause reproductive abnormalities in female rats, this concentration is also showed some of the reproductive defromalities. However it is very clear from results that both 50 mg and 100 mg BC-A/kg body wt. exposed females had remarkable deformalities in reproduction. The docking studies are in support of the above, where the β-ER and aromatse are competitively inhibited by phytoestrogen BC-A, which explains the decreased reproductive performance in the females in this study. Furthermore the inhibitory studies at molecular level are needed to know more about BC-A induced suppressed female reproduction in rats.
Acknowledgments
The authors thank Mr. Rajesh, research student, Sastara University, Thanjavur for helping in computational docking studies and its analysis. Authors are also thankful to the funding agencies University Grants Commission, New Delhi (F. No. 41-582/2012 (SR), Dated: 18.07.2012) and the Department of Science and Technology- SERB, New Delhi (No. SR/FT/ LS-180/2009, Dated: 30-04-2012) for partial support for this research in the form of research grants to Dr. P. Ramachandra Reddy, Department of Biochemistry, Yogi Vemana University, Kadapa .
References
- Cos P, De Bruyne T, Apers S, VandenBerghe D, Pieters L, et al. (2003) Phytoestrogens: recent developments.Planta Med 69: 589-599.
- Price KR, Fenwick GR (1985) Naturally occurring oestrogens in foods--a review. Food AdditContam 2: 73-106.
- Knight DC, Eden JA (1996) A review of the clinical effects of phytoestrogens. Obstet Gynecol 87: 897-904.
- Cavaliere C, Cucci F, Foglia P, Guarino C, Samperi R, et al. (2007) Flavonoid profile in soybeans by high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 21: 2177-2187.
- Setchell KD, Brown NM, Zimmer-Nechemias L, WT Brashear, Wolfe BE, et al. (2002) Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J ClinNutr 76: 447-453.
- Tomar RS, Shiao R (2008) Early life and adult exposure to isoflavones and breast cancer risk. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 26: 113-173.
- Hur H, Rafii F (2000) Biotransformation of the isoflavonoidsbiochanin A, formononetin, and glycitein by Eubacteriumlimosum. FEMS Microbiol Lett 192: 21-25.
- Bovee TF, Helsdingen RJ, Rietjens IM, Keijer J, Hoogenboom RL (2004) Rapid yeast estrogen bioassays stably expressing human estrogen receptors alpha and beta, and green fluorescent protein: a comparison of different compounds with both receptor types. J Steroid Biochem Mol Biol 91: 99-109.
- Zhu BT, Han GZ, Shim JY, Wen Y, JiangXR (2006a) Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology 147: 4132-4150.
- Brzezinski A, Debi A (1999) Phytoestrogens: the "natural" selective estrogen receptor modulators? Eur J Obstet Gynecol Reprod Biol 85: 47-51.
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA (1996) Cloning of a novel receptor expressed in rat prostate and ovary. ProcNatlAcadSci USA 93: 5925-5930.
- Mosselman S, Polman J, Dijkema R (1996) ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett 392: 49-53.
- Adlercreutz H (1998) Evolution, nutrition, intestinal microflora, and prevention of cancer: a hypothesis. ProcSocExpBiol Med 217: 241-246.
- Santti R, Mäkelä S, Strauss L, Korkman J, Kostian ML (1998) Phytoestrogens: potential endocrine disruptors in males. Toxicol Ind Health 14: 223-237.
- Horn-Ross PL, Barnes S, Lee M, Coward L, Mandel JE, et al. (2000) Assessing phytoestrogen exposure in epidemiologic studies: Development of a database (United States). Cancer Causes Control 11: 289-298.
- Mazur WM, Duke JA, Wahala K, Rasku S, Adlercreutz H (1998) Isoflavonoids and lignans in legumes: nutritional and health aspects in humans. J NutrBiochem 9: 193-200.
- Lee YS, Seo JS, Chung HT, Jang JJ (1991) Inhibitory effects of biochanin A on mouse lung tumor induced by benzo(a)pyrene. J Korean Med Sci 6: 325-328.
- Suthar AC, Banavalikar MM, Biyani MK (2001) Pharmacological activities of Genistein, an isoflavone from soy (Glycine max): part II-anti-cholesterol activity, effects on osteoporosis and menopausal symptoms. Indian Journal of Experimental Biology 39: 520-525.
- Awoniyi CA, Roberts D, Veeramachaneni DN, Hurst BS, Tucker KE, et al. (1998) Reproductive sequelae in female rats after in utero and neonatal exposure to the phytoestrogen genistein. FertilSteril 70: 440-447.
- Nagao T, Yoshimura S, Saito Y, Nakagomi M, Usumi K, et al. (2001) Reproductive effects in male and female rats of neonatal exposure to genistein. Reprod Toxicol 15: 399-411.
- Jefferson WN, Padilla-Banks E, NewboldRR (2005) Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol Reprod 73: 798-806.
- Gotoh T, Yamada K, Yin H, Ito A, Kataoka T, et al. (1998) Chemoprevention of N-nitroso-N-methylurea-induced rat mammary carcinogenesis by soy foods or biochanin A. Jpn J Cancer Res 89: 137-142.
- Lee BJ, JungEY, Yun YW, Kang JK, Baek IJ, et al. (2004), Effects of exposure to genistein during pubertal development on the reproductive system of male mice. J Reprod Dev 50: 399-409.
- Rice L, Samedi VG, Medrano TA, Sweeney CA, Baker HV, et al. (2002) Mechanisms of the growth inhibitory effects of the isoflavonoidbiochanin A on LNCaP cells and xenografts. Prostate 52: 201-212.
- Atkinson C, Compston JE, Day NE, Dowsett M, Bingham SA (2004) The effects of phytoestrogen isoflavones on bone density in women: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 79: 326-333.
- Schwartz H, Sontag G, Plumb J (2009) Inventory of phytoestrogen databases. Food Chem 113: 736-747.
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, et al. (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138: 863-870.
- Casanova M, You L, Gaido KW, Archibeque-Engle S, Janszen DB, et al. (1999) Developmental effects of dietary phytoestrogens in Sprague-Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro. Toxicol Sci 51: 236-244.
- Pfitscher A, Reiter E, Jungbauer A (2008) Receptor binding and transactivation activities of red clover isoflavones and their metabolites. J Steroid Biochem Mol Biol 112: 87-94.
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, et al. (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139: 4252-4263.
- Bowe J, Li XF, Sugden D, Katzenellenbogen JA, Katzenellenbogen BS, et al. (2003) The effects of the phytoestrogen, coumestrolon gonadotrophin releasing hormone (GnRH) m-RNA expression in GT1-7 GnRH neurons. J NeuroEndocrinol 15: 105-108.
- Long JA, Evans HM (1922) The oestrous cycle in the rat and its associated phenomena. Mem Univ Calif 6: 1-148.
- Freeman ME (1988) The ovarian cycle of the rat. In: Knobil E, Neil J (eds.). Physiology of reproduction. New York, Raven Press Ltd, pp: 1893-928.
- Young WC, Boling JL, Blandau R (1941) The vaginal smear picture, sexual receptivity and time of ovulation in the albino rat. Anat Rec 80: 37-45.
- Schwartz Nb (1964) Acute effects of ovariectomy on pituitary lh, uterine weight, and vaginal cornification. Am J Physiol 207: 1251-1259.
- Mandl AM (1951) The phases of the oestrous cycle in the adult white rat. J Exp Biol 28: 576-84.
- Zarrow MX, JM Yochim, McCarthyJL (1964) Experimental endocrinology: A sourcebook of basic techniques, New York.
- Cooper RL, Goldman JM, Vandenbergh JG (1993) Monitoring of the estrous cycle in the laboratory rodent by vaginal lavage. Methods in Toxicology: Female Reproductive Toxicology. New York, Academic Press, pp: 45-54.
- Hood RD (1997) Hand book of developmental toxicology. New York: CRC Press.
- Fisher JS (2004) Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction 127: 305-315.
- Murkies AL, Wilcox G, Davis SR (1998) Clinical review 92: Phytoestrogens. J Clin Endocrinol Metab 83: 297-303.
- Khan MA (1981) Reproduction and growth of progeny of female mice mated after treatment with crufomate. J Environ Sci Health B 16: 141-157.
- Fish SA (1966) Organophosphorus cholinesterase inhibitors and fetal development. Am J Obstet Gynecol 96: 1148-1154.
- Soratur SM, Kaliwal BB (1998) Effect of methyl parathion on pregnancy in albino Rats. Ecol Env Cons 4: 145-149.
- Lephart ED, West TW, Weber KS, Rhees RW, Setchell KD, et al. (2002) Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol 24: 5-16.
- Lephart ED, Setchell KD, Lund TD (2005) Phytoestrogens: hormonal action and brain plasticity. Brain Res Bull 65: 193-198.
- Adler JH, Trainin DA (1960) Hyperoestrogenic syndrome in cattle. Refuah Vet 17: 115.
- Kouki T, Kishitake M, Okamoto M, Oosuka I, Takebe M, et al. (2003) Effects of neonatal treatment with phytoestrogens, genistein and daidzein, on sex difference in female rat brain function: estrous cycle and lordosis. Horm Behav 44: 140-145.
- Nikaido Y, Yoshizawa K, Danbara N, Tsujita-Kyutoku M, Yuri T, et al. (2004) Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod Toxicol 18: 803-811.
- Bateman HL, Patisaul HB (2008) Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptinfiber density in the hypothalamus. Neurotoxicology 29: 988-997.
- Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, et al. (2009) Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Human Repro Update 15: 423-440.
- Losa SM, Todd KL, Sullivan AW, Cao J, Mickens JA, et al. (2011) Neonatal exposure to genistein adversely impacts the ontogeny of hypothalamic kisspeptinsignaling pathways and ovarian development in the peripubertal female rat. Reprod Toxicol 31: 280-289.
- Henry LA, Witt DM (2002) Resveratrol: phytoestrogen effects on reproductive physiology and behavior in female rats. Horm Behav 41: 220-228.
- Delclos KB, Weis CC, Bucci TJ, Olson G, Mellick P, et al. (2009) Overlapping but distinct effects of genistein and ethinylestradiol (EE2) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reproductive Toxicology 27: 117-132.
- Jefferson WN, Doerge D, Padilla-Banks E, Woodling KA, Kissling GE, et al. (2009a) Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environmental Health Perspectives 117: 1883-1889.
- Murthy DR, Reddy CM, Patil SB (1997) Effect of benzene extract of Hibiscus rosasinensis on the estrous cycle and ovarian activity in albino mice. Biol Pharm Bull 20: 756-758.
- Pushpalath T (2004) In utero exposure of proluton depot on male reproduction in rats. Thesis submitted to Sri Venkateswara University, Tirupati, A.P., and India.
- Jefferson WN, Newbold RR, Padilla-Banks E, Pepling M (2006) Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biology of Reproduction 74: 161-168.
- Jefferson WN, Padilla-Banks E, Goulding EH, Lao SP, Newbold RR, et al. (2009b) Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support pre-implantation embryo development and implantation. Biology of Reproduction 80: 425-431.
- Henke BR, Consler TG, Go N, Hale RL, Hohman DR, et al. (2002) A new series of estrogen receptor modulators that display selectivity for estrogen receptor beta. J Med Chem 45: 5492-5505.
- Doerksen T, Benoit G, Trasler JM (2000) Deoxyribonucleic acid hypomethyla-tion of male germ cells by mitotic and meiotic exposure to 5-azacytidine is associated with altered testicular histology. Endocrinology 141: 3235-3244.
- Kelly TL, Li E, Trasler JM (2003) 5-aza-2'-deoxycytidine induces alterations in murine spermatogenesis and pregnancy outcome. J Androl 24: 822-830.
- Davis SR, Murkies AL, Wilcow G (1998) Phytoestrogens in clinical practice. Integr Med 1: 27-34.
- Yoshinaga K, Adams CE (1966) Delayed implantation in the spayed, progesterone treated adult mouse. J Reprod Fertil 12: 593-595.
- Psychoyos A (1973)Endocrine control of egg implantation. Vol.IX. In. R.O. Greep, E.B. Astwood, Gieger (eds.). Hand book of physiology. American Physiological society Washington, pp: 187-215.
- Huet YM, Dey SK (1987) Role of early and late oestrogenic effects on implantation in the mouse. J Reprod Fertil 81: 453-458.
- Saraiya M, Berg CJ, Kendrick JS, Strauss LT, Atrash HK, et al. (1998) Cigarette smoking as a risk factor for ectopic pregnancy. Am J Obstet Gynecol 178: 493-498.
- Almeida FC, Lemonica IP (2000) The toxic effects of Coleus barbatus B. on the different periods of pregnancy in rats. J Ethnopharmacol 73: 53-60.
- Chang CV, Felício AC, Reis JE, Guerra Mde O, Peters VM (2002) Fetal toxicity of Solanumlycocarpum (Solanaceae) in rats. J Ethnopharmacol 81: 265-269.
- Dabhadkar D, Zade V (2012) Abortifacient activity of Plumeriarubra (Linn) pod extract in female albino rats. Indian J Exp Biol 50: 702-707.
- Yakuba MT, Adeshina AO, Oladiji AT, Akanji MA, Oloyede OB, et al. (2010) Abortifacient potential of aqueous extract of sennaalata leaves in rats. J Reprodcotracep 121: 163.
- Elbetieha A, Oran SA, Alkofahi A, Darmani H, Raies AM (2000) Fetotoxic potentials of Globulariaarabica and Globulariaalypum (Globulariaceae) in rats. J Ethnopharmacol 72: 215-219.
- Hsieh, C.Y., Santell, R.C., Haslam, S.Z., Helferich, W.G. (1998) Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer research 58: 3833.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences