Congenital Iodide Transport Defect: Recent Advances and Future Perspectives
Mariano Martín and Juan Pablo Nicola
Clinical Research Center and Immunology Biochemistry - National Council of Scientific and Technical Research ( CONICET CIBICI ), Department of Clinical Biochemistry, Faculty of Chemistry, National University of Cordoba, Haya de la Torre and Medina Allende, Córdoba, Argentina
- *Corresponding Author:
- Nicola JP
Clinical Research Center and Immunology Biochemistry - National Council of Scientific and Technical Research ( CONICET CIBICI )
Department of Clinical Biochemistry
Faculty of Chemistry
National University of Cordoba
Haya de la Torre and Medina Allende
5000 Córdoba, Argentina
Tel: +54 0351 535-3851
E-mail: jpnicola@fcq.unc.edu.ar
Received date: May 21, 2016; Accepted date: June 11, 2016; Published date: June 13, 2016
Citation: Martin M, Nicola JP (2016) Congenital Iodide Transport Defect: Recent Advances and Future Perspectives. J Clin Mol Endocrinol 1:9 doi: 10.21767/2572-5432.100010
Copyright: © 2016 Martin M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Iodide is an irreplaceable component of thyroid hormones; therefore, a key requirement for thyroid hormone synthesis is that iodide is actively accumulated in the thyroid. The ability of thyroid follicular cell to concentrate iodide relies on the functional expression of the sodium/iodide symporter (SIS) at the plasma membrane. Underscoring the significance of SIS for thyroid physiology, naturally occurring loss-of-function SIS mutations cause iodide transport defect (ITD) autosomalrecessive disorders in which iodide accumulation is severely or totally impaired, leading to dyshormonogenic congenital hypothyroidism. Up to date, sixteen different loss-of-function mutations in the gene encoding SIS have been reported. Surprisingly, marked clinical heterogeneity between patients harboring the different (or even the same) SIS mutation without a clear genotype–phenotype correlation has been observed. Residual mutant SIS activity and iodide intake levels have been proposed to explain the difference in the age of onset on hypothyroidism and the development of goiter. Significantly, genetic screening is highly recommended in patients with severely reduced radioiodide accumulation even in the absence of goiter. The identification of mutations in SIS may allow subsequent preclinical diagnoses of younger members of the family as patients with delayed onset on hypothyroidism had already signs of developmental delay at time of diagnosis. Moreover, iodide supplementation can improve thyroid function in patients with residual SIS activity and should be considered. This review summarizes the current knowledge regarding the molecular basis of ITD, as well as the clinical and biochemical presentation of patients with ITD. Moreover, we explore the latest advances in the molecular characterization of ITD-causing slc5a5 mutants whose study has yielded invaluable information into the molecular mechaSISm of SIS and the perspectives of understanding naturally occurring SIS mutants to improve radioiodide therapy in thyroid cancer as well as SIS-based gene therapy.
Keywords
Thyroid hormone; Congenital hypothyroidism; Iodide transport defect; Thyroid scintigraphy; Sodium iodide symporter; Radioiodide therapy
The Significance of Dietary Iodide
Iodide accumulation in the thyroid gland constitutes the first step for thyroid hormones synthesis, the only iodinecontaining hormones in vertebrates. Thyroid hormones are essential for normal growth and development of a variety of organ systems, especially the central nervous system [1]. As iodine is an irreplaceable constituent of the thyroid hormones, normal thyroid physiology relies on adequate dietary iodide intake, gastro-intestinal iodide absorption, and proper iodide accumulation in the thyroid follicular cell.
Iodine is extremely scarce in the environment and is supplied to the body exclusively through the diet. Dietary iodide deficiency during childhood may cause mild to severe hypothyroidism and subsequently goiter, stunted growth, retarded psychomotor development, cognitive impairment, and even irreversible mental retardation due to thyroid hormone deficiency [2]. Significantly, iodide deficiency disorders are the most common preventable cause of mental retardation in the world. Although iodine deficiency prevention programs by iodination of table salt have virtually eliminated iodide deficiency in several countries, nearly 2 billion individuals have insufficient iodine intake and remain on risk to develop iodide deficiency disorders [3].
Congenital Hypothyroidism
Congenital hypothyroidism, defined as thyroid hormone deficiency present at birth, is the most common inborn endocrine disorder with an incidence estimated in 1:3,000-4,000 newborns. In countries with sufficient iodide nutrition status, 80-85% of congenital hypothyroidism is caused by genetic abnormalities resulting in thyroid dysgenesis —agenesis, hypoplasia, ectopy, whereas 10-15% are the consequence of a genetic defect impairing one of the steps involved in thyroid hormone synthesis, also called dyshormonogenesis [4]. Other less common causes are central hypothyroidism—absence of thyroid gland stimulation due to a deficiency of thyroid stimulating hormone (TSH)—or defects in peripheral thyroid hormone action, cell transport, and metabolism [5,6].
Congenital hypothyroidism causes stunted growth and irreversible mental retardation without early and adequate replacement therapy. Therefore, current newborn screening programs primarily seek to detect elevated TSH levels at birth in response to deficient thyroid hormone production or action that enable an early onset of levothyroxine therapy. Although most patients with congenital hypothyroidism develop successfully after levothyroxine replacement, patients with defects in the metabolism of thyroid hormones cannot be treated with levothyroxine alone [7]. Nowadays, the design of new treatment options for patients with these rare conditions is one of the most challenging tasks in the field of congenital thyroid diseases.
Iodide Transport Defect (ITD)
ITD is an uncommon autosomal-recessive disorder characterized by the inability of the thyroid follicular cell to actively accumulate iodide, thus leading to dyshormonogenic congenital hypothyroidism due to the scarcity of iodide [8]. The biochemical presentation of ITD is characterized, when untreated, by hypothyroidism and enlargement of the thyroid gland of different degree, normal to increased serum thyroglobulin levels, severely reduced to absent radioiodide accumulation in the thyroid and salivary glands, low iodide saliva-to-plasma ratio (normal value>10), and high iodide cerebrospinal fluid-to-plasma ratio (normal value approximately 0.04) [9]. Significantly, the clinical presentation of the resulting phenotypes varies from euthyroid to severe hypothyroidism [8].
In patients with ITD, thyroid scintigraphy reveals severely reduced to absent radioiodide accumulation in a normally located thyroid gland. A normal thyroid gland accumulates about 10-40% of ingested radioiodide, while in patients with ITD it accumulates 0–5% and thyroid scintigraphy may suggest thyroid dysgenesis especially when the presence of the thyroid gland is missed by ultrasound analysis.
Since the absence of radioiodide accumulation is generalized, deficient radioiodide accumulation is also observed in salivary glands and stomach when whole body scans are performed. Thyroid ultrasound examination and serum thyroglobulin levels further helps to distinguish ITD from other conditions with reduced iodide uptake as they show, characteristically, a normally located thyroid gland and normal to high thyroglobulin levels, excluding thyroid gland dysgenesis or TSH receptor signaling defects.
The introduction of radioactive iodide isotopes into the study of thyroid physiology [10] has been a cornerstone in the identification of goitrous patients with a defect in the accumulation of iodide in the thyroid tissue. Interestingly, the first report in the literature describing a patient with an ITD phenotype was reported as early as 1957 [11].
However, it was not until the isolation of the complementary DNA encoding the transporter mediating the active accumulation of iodide in the thyroid tissue—the sodium iodide symporter (SIS)—that the cause of ITD was elucidated for the first time at the molecular level.
Sodium Iodide Symporter (SIS)
Active iodide accumulation in the thyroid tissue is mediated by SIS, an integral plasma membrane glycoprotein located on the basolateral surface of the thyroid follicular cell [12]. SIS represents a highly specialized transport system mediating the accumulation of iodide from the bloodstream into the thyroid follicular cell as well as a number of extra-thyroidal tissues, including lactating breast, salivary glands, stomach, small intestine, and choroid plexus [13-17].
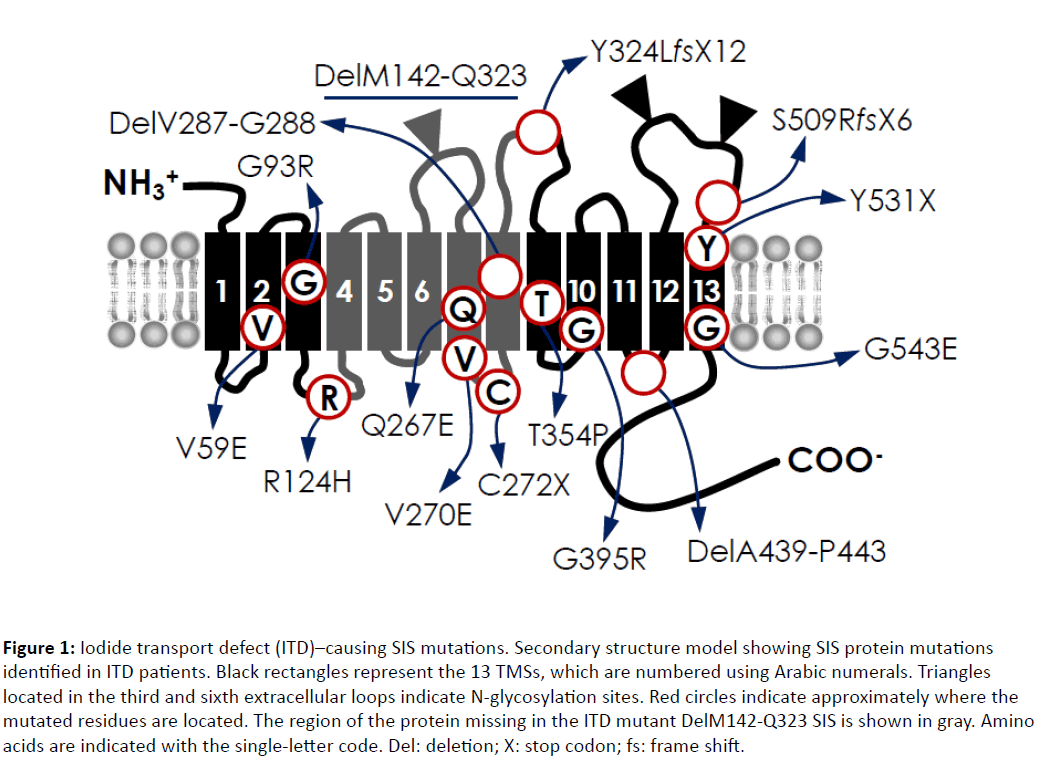
The human slc5a5 gene, which encodes SIS, is located on chromosome 19p12–13 with an open reading frame of 1,929 nucleotides encoding a 643-amino acid protein with a molecular mass of approximately 50 kDa [18]. The coding region of human SIS comprises 15 exons spanning 23.2 kb, encoding for a 3.9 kb mRNA [18,19]. The current experimentally tested SIS secondary structure model predicts a protein with 13 transmembrane segments (TMSs), an extracellular amino-terminus and a large intracellular carboxylterminus (Figure 1) [20,21]. This model has been reinforced by the determination of the crystal structure of the Vibrio parahaemolyticus.
Figure 1: Iodide transport defect (ITD)–causing SIS mutations. Secondary structure model showing SIS protein mutations identified in ITD patients. Black rectangles represent the 13 TMSs, which are numbered using Arabic numerals. Triangles located in the third and sixth extracellular loops indicate N-glycosylation sites. Red circles indicate approximately where the mutated residues are located. The region of the protein missing in the ITD mutant DelM142-Q323 SIS is shown in gray. Amino acids are indicated with the single-letter code. Del: deletion; X: stop codon; fs: frame shift.
Na+/galactose transporter (vSGLT), a bacterial homologue of the human SGLT1 [22]. Remarkably, SIS shares 27% identity and 58% homology with vSGLT—almost as much as SGLT1 does (31% identity, 62% homology). Therefore, using the crystal structure of vSGLT as a template, Paroder-Belenitsky et al. [23] generated a 3D homology model of SIS. Significantly, the SIS homology model has been extremely valuable in the functional characterization of ITD-causing SIS mutants.
The protein sequence of human SIS presents three cores Nglycosylation sites at N225, N489, and N502 located in the third and sixth extracellular loops (Figure 1). All three sites are N-glycosylated turning SIS into a highly glycosylated protein [21]. The electrophoretic pattern of SIS comprises partially glycosylated (60 kDa) and fully glycosylated (100 kDa) polypeptides. Fully glycosylated SIS is the functional polypeptide targeted to the plasma membrane. However, in non-polarized cells, N-glycosylation is not critical for SIS activity or its targeting to the plasma membrane [21,24].
SIS transports one iodide ion against its electrochemical gradient together with two sodium ions along their electrochemical gradient maintained by the activity of Na+/K+- ATPase [25]. Although the main known physiological role of SIS function is to transport iodide, SIS has also been demonstrated to transport other structurally similar anions [25]. Significantly, SIS mediates the accumulation of 99mTc-pertechnetate and, therefore, it is widely used for thyroid scintigraphy [26]. However, unlike iodide, which is both accumulated and covalently incorporated into thyroglobulin in the thyroid, pertechnetate is only accumulated in the thyroid. Another clinically relevant SIS substrate is the environmental pollutant and well known inhibitor of thyroidal iodide uptake perchlorate. The blockage of iodide transport by perchlorate has been used in the treatment of hyperthyroidism, and is currently used in the perchlorate discharge test to evaluate iodide organification defects [27].
Studying the mechaSISm of SIS-mediated perchlorate transport, Dohán et al. [28] uncovered that SIS translocates different substrates with different stoichiometries, a property that had not been reported in any other transporter. The authors evaluated the kinetic parameters of SIS-mediated perchlorate transport using the structurally related anion 186Re-perrhenate. The initial rates of perrhenate transport—as a function of extracellular Na+—yielded a hyperbolic curve indicative of an electroneutral stoichiometry (1 sodium/1 perrhenate or perchlorate), which stands in stark contrast to the electrogenic (2 sodium/1 iodide) transport of iodide [28].
Iodide accumulation in the thyroid follicular cell is a remarkable process given that the concentration of iodide in the blood is in the sub-micromolar range—well below the affinity of SIS for iodide—and the difficulty of binding halide ions with high affinity. Using statistical thermodynamics, Nicola et al. [29] reported that SIS overcomes this problem by taking advantage of the extracellular concentration of sodium and the increase in affinity for iodide that occurs when the sodium binds to the transporter. Significantly, at the physiological concentration of sodium, 79% of SIS molecules are occupied by two sodium ions, and hence poised to bind and transport iodide [29].
Genetic Causes of Iodide Transport Defect (ITD)
Shortly after the cloning of SIS [12], the first loss-of-function mutation in slc5a5 was described in a Japanese patient diagnosed with congenital hypothyroidism due to a failure to concentrate radioiodide in the saliva and a clinical response to potassium iodide treatment [30]. Up to date, patients with ITD were found to be homozygous or compound heterozygous for sixteen different loss-of-function mutations in the slc5a5 gene (-54C>T, V59E, G93R, R124H, Q267E, V270E, C272X, Y324LfsX12, T354P, G395R, S509RfsX6, Y531X, G543E, DelM143-Q323, DelV287-G288, and DelA439-P443) (Figure 1). They are either nonsense, alternative splicing, frame-shift, deletion, or missense mutations of the slc5a5 gene. Significantly, no mutation in other genes related to SIS function or targeting to the plasma membrane have been reported as cause of ITD.
As shown in Figure 1, most ITD-causing SIS mutants have been reported within the open reading frame encoding the transporter. In addition, a mutation in the 5’ untranslated region of SIS, a C to T transition at nucleotide -54 (-54C>T) that reduces SIS translation efficiency with a subsequent decrease in the functional expression of the transporter, has been reported [31].
The clinical evaluation of patients with different loss-offunction slc5a5 mutations has demonstrated a substantial clinical and biochemical heterogeneity. The onset of hypothyroidism has varied from birth to childhood and seems to correlate with the residual mutant SIS activity across slc5a5 defects [8]. Significantly, less than 10% of patients with neonatal screening-detected ITD evidenced clinical signs of hypothyroidism [8]. The presence of residual mutant SIS activity, which is compatible with the presence of reduced, although not absent, radioiodide uptake, revealed by thyroid scintigraphy constitutes a possible explanation for the difference in the age of onset on hypothyroidism. In this context, the increase in TSH levels following a reduction in thyroid hormone production may partially overcome the defect in mutant SIS function by enhancing SIS expression, as occurred in patients with ITDs carrying the missense mutation T354P [32]. In agreement, a TSH-induced overstimulation of the thyroid gland results in high serum thyroglobulin levels [33]. In sharp contrast, patients harboring homozygous fully inactive SIS mutants developed hypothyroidism with significant clinical manifestations as a neonate [8]. Moreover, a marked clinical heterogeneity has been reported in patients harboring the same SIS mutant, especially concerning onset and severity of hypothyroidism and the development of goiter. Indeed, the clinical heterogeneity was first reported in a large series of patients carrying the SIS mutant T354P [34]. The amount of iodide intake significantly influences thyroid function in patients with ITD, especially in those whose mutant SIS protein retain residual activity. Breast milk contains much larger amounts of iodide than artificial milk, particularly in lactating mothers who take large amounts of seafood, especially seaweed. Significantly, marked differences in hypothyroidism onset were noticed between siblings feed with breast milk or artificial milk during infancy [34].
Molecular Characterization of ITDCausing SIS Mutants
The detailed molecular study of ITD-causing SIS mutants has provided key mechaSIStic information on SIS structure/ function; in particular, residues have been identified that are critical for substrate binding, specificity, stoichiometry, and plasma membrane targeting.
The molecular analysis of the SIS mutant T354P—the first ITD-causing SIS mutant identified—revealed that SIS function requires a hydroxyl group at the β-carbon at this position [35], and led to the study of other β-hydroxyl group-containing residues in TMS IX. De la Vieja et al. [36] demonstrated that substitutions at position 354 and neighboring β-hydroxyl group-containing residues modify the apparent affinity of the protein for sodium, suggesting that these residues are involved in sodium coordination and translocation. The authors proposed the existence of a putative structural homology between SIS and the Aquifex aeolicus leucine transporter (LeuT)—the first sodium-driven transporter whose structure was solved by X-ray crystallography, despite a lack of primary sequence homology [36]. Consistent with this prediction, the crystal structure of several sodium-coupled transporters, including the Vibrio parahaemolyticus sodium/galactose transporter (vSGLT), which belongs to the same family as SIS [sodium/solute cotransporter family 5A (slc5a5)] [22], revealed that these transporters shared the same fold as LeuT. Surprisingly, although the transporters share little sequence homology, all share a similar mechaSISm for sodium coordination, thus evidencing a structural convergent evolutionary process for sodium-driven transporters.
Paroder-Belenitsky et al. [23] have uncovered unexpected mechaSIStic information on SIS by the detailed molecular characterization of the ITD-causing G93R SIS mutant. The lack of activity of the G93R SIS mutant was found not to be due to impaired trafficking to the plasma membrane or to the positive charge conferred by Arg in the middle of TMS 3. Instead, certain amino acid replacements at this position caused a significant change in the apparent affinity for iodide. In addition, position 93 showed to be critical for substrate stoichiometry and specificity as G93N/T/Q/E SIS mediates electrogenic transport of perrhenate and perchlorate, in contrast to the electroneutral stoichiometry of wild-type SIS; and although G93E and Q SIS discriminate between substrates, as they do not transport iodide but they mediate electrogenic transport of perrhenate and perchlorate. Furthermore, based on the SIS 3D homology model, Paroder-Belenitsky et al. [23] proposed that G93 acts as a pivot in going from an outwardly to an inwardly open conformation during the transport cycle.
The thorough characterization of the ITD-causing SIS mutations R124H and DelA439-P443 has demonstrated the importance of intramolecular interactions for proper SIS folding [24,37]. Paroder et al. [37] reported that R124H SIS is retained intracellularly in the endoplasmic reticulum and, surprisingly, intrinsically active. As a result, R124H SIS is not targeted to the plasma membrane and therefore does not mediate any iodide transport in transfected cells. Significantly, amino acid substitutions at position 124, located in intracellular loop 2 (IL-2), revealed a key structural role for the δ-amino group of R124, a highly conserved residue throughout the slc5a5 family, in SIS targeting to the plasma membrane. Indeed, an intramolecular interaction between the δ-amino group of R124 and the thiol group of C440, located in IL-6, is critical for the local folding required for SIS sorting out through endoplasmic reticulum quality-control system [37]. Li et al. demonstrated that DelA439-P443 SIS is also retained in the endoplasmic reticulum but intrinsically inactive [24]. To study the defect caused by the deleted residues, the authors engineered five consecutive Ala residues at positions A439– P443, which partially recovered plasma membrane targeting. Strikingly, when only N441 was restored in the five Ala background, the resulting SIS protein was fully targeted to the plasma membrane [24]. Using the SIS 3D homology model, the authors proposed that the side chain of N441—a residue conserved throughout most of the slc5a5 family members, interacts with the main chain amino group of G444, capping the helix of TMS 12 and thus stabilizing the structure of the molecule.
Very recently, Nicola et al. [38] reported novel compound heterozygous loss-of-function SIS mutations—R124H and V270E—as cause of ITD in a Jamaican pediatric patient with a peculiar phenotype: stunted growth but no cognitive deficiency. According to the 3D SIS homology model, V270 is placed at the intracellular end of TMS 7. The authors showed that V270E markedly reduces iodide uptake when the protein is heterologously expressed, because targeting of V270E SIS to the plasma membrane is severely impaired. Strikingly, membrane vesicles from V270E SIS-expressing cells transported as much iodide as membrane vesicles from wildtype SIS expressing cells, indicating that the mutant protein is intrinsically active, thus a negative charge at position 270 does not destabilize the protein but rather interferes its trafficking to the plasma membrane [38]. The negative charge imparted by Glu at position 270 induced a subtle change in the surface charge of a positive patch in the intracellularly facing domain of the mutant SIS molecule that may hinder the interaction with proteins critical for its trafficking to the cell surface [38]. In conclusion, a nonpolar residue at position 270, which all members of the slc5a5 family have, is required for proper SIS maturation and plasma membrane trafficking.
Concluding Remarks
Genetic screening should be considered in all pediatric patients with permanent hypothyroidism in conjunction with low or absent radioiodide uptake even in the absence of goiter. In case that thyroid scintigraphy data is not available, patients with goitrous hypothyroidism associated with high serum thyroglobulin levels should be first evaluated for organification defects. In addition to providing a definitive diagnosis, the identification of mutations in slc5a5 may have further clinical implications. Genetic diagnosis of a proband will allow subsequent preclinical diagnoses of younger siblings in the same family as patients with delayed onset on clinical hypothyroidism in ITD had already signs of developmental delay at time of diagnosis [38]. Moreover, concerning treatment options, iodide supplementation can improve thyroid function in patients with residual SIS activity and should be considered, either as adjunct or alternative treatment to levothyroxine replacement.
In addition to the fundamental role of SIS for thyroid hormone synthesis, SIS-mediated iodide accumulation in the thyroid gland constitutes the molecular basis for the diagnosis and treatment of thyroid cancer based on radioiodide therapy [39]. For over 70 years, radioiodide therapy has been the mainstay therapy for radioiodide-avid locally advanced and metastatic thyroid carcinomas. Retrospective studies have demonstrated that the ability of tumor cells to accumulate radioiodide is the best indicator of disease-free survival [40]. However, differentiated thyroid tumors often exhibit reduced (or even undetectable) iodide transport compared to normal thyroid tissue. Immunohistochemical analysis revealed that 70-80% of thyroid tumors overexpress SIS compared to adjacent normal tissue. Surprisingly, SIS expression was mainly located in intracellular compartments suggesting the presence of cell surface trafficking abnormalities. The mechaSISms leading to reduced SIS trafficking to the plasma membrane in thyroid tumors constitutes still a major open question in the thyroid field. Significantly, considering the substantial information regarding SIS structure/function obtained through the study of naturally occurring SIS mutants, the identification and further molecular characterization of novel ITD-causing SIS mutants supposes a promise strategy to uncover the mechaSISms mediating SIS targeting to the plasma membrane in thyroid follicular cells.
Acknowledgment
We are grateful to Dr. Ana Maria Masini-Repiso for helpful comments on the manuscript. We also thank the members of our laboratory for critical discussion. This work was supported by grants from the Latin American Thyroid Society, the Thyroid Cancer Survivors' Association - American Thyroid Association, and the Agencia Nacional de Promoción Científica y Tecnológica.
References
- Mullur R, Liu YY, Brent GA (2014) Thyroid hormone regulation of metabolism. Physiol Rev 94: 355-382.
- Zimmermann MB, Boelaert K (2015) Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol 3: 286-295.
- Andersson M, Karumbunathan V, Zimmermann MB (2012) Global iodine status in 2011 and trends over the past decade. J Nutr 142: 744-750.
- Wassner AJ, Brown RS (2015) Congenital hypothyroidism: recent advances. CurrOpinEndocrinol Diabetes Obes 22: 407-412.
- Refetoff S, Bassett JH, Beck-Peccoz P, Bernal J, Brent G, et al.(2014) Classification and proposed nomenclature for inherited defects of thyroid hormone action, cell transport, and metabolism. J ClinEndocrinolMetab99:768-770.
- Schoenmakers N, Alatzoglou KS, Chatterjee VK, Dattani MT (2015) Recent advances in central congenital hypothyroidism. J Endocrinol 227: R51-71.
- Krude H, Kühnen P, Biebermann H (2015) Treatment of congenital thyroid dysfunction: Achievements and challenges. Best Pract Res ClinEndocrinolMetab 29: 399-413.
- Szinnai G, Kosugi S, Derrien C, Lucidarme N, David V, et al.(2006) Extending the clinical heterogeneity of iodide transport defect (ITD): a novel mutation R124H of the sodium/iodide symporter gene and review of genotype-phenotype correlations in ITD. J ClinEndocrinolMetab91:1199-1204.
- Wolff J (1983) Congenital goiter with defective iodide transport. Endocr Rev 4: 240-254.
- Hertz S, Roberts A, Means JH, Evans RD (1940) Radioactive iodine as an indicator in thyroid physiology. Am J Physiol 128:565-576.
- federman D, Robbins J, Rall Je (1958) Some observations on cretinism and its treatment. N Engl J Med 259: 610-615.
- Dai G, Levy O, Carrasco N (1996) Cloning and characterization of the thyroid iodide transporter. Nature 379: 458-460.
- Wapnir IL, van de Rijn M, Nowels K, Amenta PS, Walton K, et al. (2003)Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J ClinEndocrinolMetab 88:1880-1888.
- Tazebay UH, Wapnir IL, Levy O, Dohan O, Zuckier LS, et al.(2000) The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med 6:871-878.
- Altorjay A, Dohán O, Szilágyi A, Paroder M, Wapnir IL, et al. (2007) Expression of the Na+/I- symporter (NIS) is markedly decreased or absent in gastric cancer and intestinal metaplastic mucosa of Barrett esophagus. BMC Cancer 7: 5.
- Nicola JP, Reyna-Neyra A, Carrasco N, Masini-Repiso AM (2012) Dietary iodide controls its own absorption through post-transcriptional regulation of the intestinal Na+/I- symporter. J Physiol 590: 6013-6026.
- Nicola JP, Basquin C, Portulano C, Reyna-Neyra A, Paroder M, et al.(2009) The Na+/I- symporter mediates active iodide uptake in the intestine. Am J Physiol Cell Physiol 296:C654-662.
- Smanik PA, Ryu KY, Theil KS, Mazzaferri EL, Jhiang SM (1997) Expression, exon-intron organization, and chromosome mapping of the human sodium iodide symporter. Endocrinology 138:3555-3558.
- Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, et al. (1996) Cloning of the human sodium lodidesymporter. BiochemBiophys Res Commun 226: 339-345.
- Levy O, Dai G, Riedel C, Ginter CS, Paul EM, et al. (1997) Characterization of the thyroid Na+/I- symporter with an anti-COOH terminus antibody. Proc Nat AcadSci USA 94: 5568-5573.
- Levy O, De la Vieja A, Ginter CS, Riedel C, Dai G, et al. (1998) N-linked glycosylation of the thyroid Na+/I- symporter (NIS). Implications for its secondary structure model. J BiolChem 273: 22657-22663.
- Faham S, Watanabe A, Besserer GM, Cascio D, Specht A, et al. (2008)The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science 321:810-814.
- Paroder-Belenitsky M, Maestas MJ, Dohán O, Nicola JP, Reyna-Neyra A, et al. (2011) Mechanism of anion selectivity and stoichiometry of the Na+/I- symporter (NIS). ProcNatlAcadSci USA 108: 17933-17938.
- Li W, Nicola JP, Amzel LM, Carrasco N (2013) Asn441 plays a key role in folding and function of the Na+/I- symporter (NIS). FASEB J 27: 3229-3238.
- Eskandari S, Loo DD, Dai G, Levy O, Wright EM, et al. (1997) Thyroid Na+/I- symporter. Mechanism, stoichiometry, and specificity. J BiolChem 272: 27230-27238.
- Franken PR, Guglielmi J, Vanhove C, Koulibaly M, Defrise M, et al.(2010) Distribution and dynamics of (99m)Tc-pertechnetate uptake in the thyroid and other organs assessed by single-photon emission computed tomography in living mice. Thyroid 20:519-526.
- Hilditch TE, Horton PW, McCruden DC, Young RE, Alexander WD (1982) Defects in intrathyroid binding of iodine and the perchlorate discharge test. ActaEndocrinol (Copenh) 100:237-244.
- Dohan O, Portulano C, Basquin C, Reyna-Neyra A, Amzel LM, et al.(2007) The Na+/I symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. ProcNatlAcadSci USA 104:20250-20255.
- Nicola JP, Carrasco N, Amzel LM (2014) Physiological sodium concentrations enhance the iodide affinity of the Na+/I- symporter. Nat Commun 5: 39-48.
- Fujiwara H, Tatsumi K, Miki K, Harada T, Miyai K, et al. (1997) Congenital hypothyroidism caused by a mutation in the Na+/I- symporter. Nat Genet 16: 124-125.
- Nicola JP, Nazar M, Serrano-Nascimento C, Goulart-Silva F, Sobrero G, et al.(2011) Iodide transport defect: functional characterization of a novel mutation in the Na+/I- symporter 5'-untranslated region in a patient with congenital hypothyroidism. J ClinEndocrinolMetab96:E1100–1107.
- Matsuda A, Kosugi S (1997) A homozygous missense mutation of the sodium/iodide symporter gene causing iodide transport defect. J ClinEndocrinolMetab 82: 3966-3971.
- Vulsma T, Rammeloo JA, Gons MH, de Vijlder JJ (1991)The role of serum thyroglobulin concentration and thyroid ultrasound imaging in the detection of iodide transport defects in infants. ActaEndocrinol (Copenh) 124:405-410.
- Kosugi S, Inoue S, Matsuda A, Jhiang SM (1998) Novel, missense and loss-of-function mutations in the sodium/iodide symporter gene causing iodide transport defect in three Japanese patients. J ClinEndocrinolMetab 83: 3373-3376.
- Levy O, Ginter CS, De la Vieja A, Levy D, Carrasco N (1998) Identification of a structural requirement for thyroid Na+/I- symporter (NIS) function from analysis of a mutation that causes human congenital hypothyroidism. FEBS Lett 429:36-40.
- De la Vieja A, Reed MD, Ginter CS, Carrasco N (2007)Amino acid residues in transmembrane segment IX of the Na+/I- symporter play a role in its Na+ dependence and are critical for transport activity. J BiolChem 282:25290-25298.
- Paroder V, Nicola JP, Ginter CS, Carrasco N (2013) The iodide-transport-defect-causing mutation R124H: a δ-amino group at position 124 is critical for maturation and trafficking of the Na+/I- symporter. J Cell Sci 126: 3305-3313.
- Nicola JP, Reyna-Neyra A, Saenger P, Rodriguez-Buritica DF, Gamez Godoy JD, et al. (2015) Sodium/Iodide symporter mutant V270E causes stunted growth but no cognitive deficiency. J ClinEndocrinolMetab 100: E1353-1361.
- Nicola JP,Masini-Repiso AM (2016)Emerging therapeutics for radioiodide-refractory thyroid cancer. J Anal Oncology5:75-86.
- Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, et al. (2006)Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J ClinEndocrinolMetab91:2892-2899.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences